Artesunate plus pyronaridine for treating uncomplicated Plasmodium falciparum malaria
- PMID: 24596021
- PMCID: PMC4448218
- DOI: 10.1002/14651858.CD006404.pub2
Artesunate plus pyronaridine for treating uncomplicated Plasmodium falciparum malaria
Update in
-
Pyronaridine-artesunate for treating uncomplicated Plasmodium falciparum malaria.Cochrane Database Syst Rev. 2019 Jan 8;1(1):CD006404. doi: 10.1002/14651858.CD006404.pub3. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2022 Jun 21;6:CD006404. doi: 10.1002/14651858.CD006404.pub4. PMID: 30620055 Free PMC article. Updated.
Abstract
Background: The World Health Organization (WHO) recommends that people with uncomplicated Plasmodium falciparum malaria are treated using Artemisinin-based Combination Therapy (ACT). ACT combines three-days of a short-acting artemisinin derivative with a longer-acting antimalarial which has a different mode of action. Pyronaridine has been reported as an effective antimalarial over two decades of use in parts of Asia, and is currently being evaluated as a partner drug for artesunate.
Objectives: To evaluate the efficacy and safety of artesunate-pyronaridine compared to alternative ACTs for treating people with uncomplicated P. falciparum malaria.
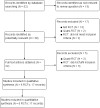
Search methods: We searched the Cochrane Infectious Diseases Group Specialized Register; Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library; MEDLINE; EMBASE; LILACS; ClinicalTrials.gov; the metaRegister of Controlled Trials (mRCT); and the WHO International Clinical Trials Search Portal up to 16 January 2014. We searched reference lists and conference abstracts, and contacted experts for information about ongoing and unpublished trials.
Selection criteria: Randomized controlled trials of artesunate-pyronaridine versus other ACTs in adults and children with uncomplicated P. falciparum malaria.For the safety analysis, we also included adverse events data from trials comparing any treatment regimen containing pyronaridine with regimens not containing pyronaridine.
Data collection and analysis: Two authors independently assessed trial eligibility and risk of bias, and extracted data. We combined dichotomous data using risk ratios (RR) and continuous data using mean differences (MD), and presented all results with a 95% confidence interval (CI). We used the GRADE approach to assess the quality of evidence.
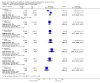
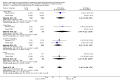
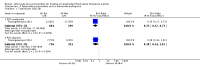
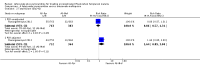
Main results: We included six randomized controlled trials enrolling 3718 children and adults. Artesunate-pyronaridine versus artemether-lumefantrineIn two multicentre trials, enrolling mainly older children and adults from west and south-central Africa, both artesunate-pyronaridine and artemether-lumefantrine had fewer than 5% PCR adjusted treatment failures during 42 days of follow-up, with no differences between groups (two trials, 1472 participants, low quality evidence). There were fewer new infections during the first 28 days in those given artesunate-pyronaridine (PCR-unadjusted treatment failure: RR 0.60, 95% CI 0.40 to 0.90, two trials, 1720 participants, moderate quality evidence), but no difference was detected over the whole 42 day follow-up (two trials, 1691 participants, moderate quality evidence). Artesunate-pyronaridine versus artesunate plus mefloquineIn one multicentre trial, enrolling mainly older children and adults from South East Asia, both artesunate-pyronaridine and artesunate plus mefloquine had fewer than 5% PCR adjusted treatment failures during 28 days follow-up (one trial, 1187 participants, moderate quality evidence). PCR-adjusted treatment failures were 6% by day 42 for these treated with artesunate-pyronaridine, and 4% for those with artesunate-mefloquine (RR 1.64, 95% CI 0.89 to 3.00, one trial, 1116 participants, low quality evidence). Again, there were fewer new infections during the first 28 days in those given artesunate-pyronaridine (PCR-unadjusted treatment failure: RR 0.35, 95% CI 0.17 to 0.73, one trial, 1720 participants, moderate quality evidence), but no differences were detected over the whole 42 days (one trial, 1146 participants, low quality evidence). Adverse effectsSerious adverse events were uncommon in these trials, with no difference detected between artesunate-pyronaridine and comparator ACTs. The analysis of liver function tests showed biochemical elevation were four times more frequent with artesunate-pyronaridine than with the other antimalarials (RR 4.17, 95% CI 1.38 to 12.62, four trials, 3523 participants, moderate quality evidence).
Authors' conclusions: Artesunate-pyronaridine performed well in these trials compared to artemether-lumefantrine and artesunate plus mefloquine, with PCR-adjusted treatment failure at day 28 below the 5% standard set by the WHO. Further efficacy and safety studies in African and Asian children are required to clarify whether this combination is an option for first-line treatment.
Figures








































References
-
- Kayentao K. Pyronaridine-artesunate versus artemether/lumefantrine: efficacy in malaria patients with uncomplicated acute falciparum malaria: results of a pivotal Phase III trial. 2008;79:114. American Journal of Tropical Medicine and Hygiene(6 Suppl)
-
- Nct00541385 Pyronaridine artesunate 3:1 granule formulation vs. Coartem© crushed tablets in P. falciparum malaria pediatric patients. www.clinicaltrials.gov/show/NCT00541385 (accessed 30 November 2010).
-
- Duparc S, Borghini-Fuhrer I, Craft JC, Arbe-Barnes S, Miller RM, Shin CS, et al. Efficacy of pyronaridine/artesunate in clinical trials in patients with uncomplicated acute Plasmodium falciparum or Plasmodium vivax malaria: results of an integrated analysis. 2009;81:51–100. American Journal of Tropical Medicine and Hygiene.
-
- Duparc S, Borghini-Fuhrer I, Craft JC, Arbe-Barnes S, Miller RM, Shin CS, et al. Safety of pyronaridine/artesunate in clinical trials in patients with uncomplicated acute Plasmodium falciparum or Plasmodium vivax malaria: results of an integrated analysis. 2009;81:101–150. American Journal of Tropical Medicine and Hygiene.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

