Synthesis and evaluation of novel dental monomer with branched carboxyl acid group
- PMID: 24596134
- PMCID: PMC4156564
- DOI: 10.1002/jbm.b.33126
Synthesis and evaluation of novel dental monomer with branched carboxyl acid group
Abstract
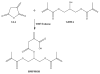
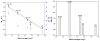
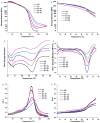
To enhance the water miscibility and increase the mechanical properties of dentin adhesives, a new glycerol-based monomer with vinyl and carboxylic acid, 4-((1,3-bis(methacryloyloxy)propan-2-yl)oxy)-2-methylene-4-oxobutanoic acid (BMPMOB), was synthesized and characterized. Dentin adhesive formulations containing 2-hydroxyethyl methacrylate (HEMA), 2,2-bis[4-(2-hydroxy-3-methacryloxypropoxy) phenyl]propane (BisGMA), and BMPMOB were characterized with regard to real-time photopolymerization behavior, water sorption, dynamic mechanical analysis, and microscale three-dimensional internal morphologies and compared with HEMA/BisGMA controls. The experimental adhesive copolymers showed higher glass transition temperature and rubbery moduli, as well as improved water miscibility compared to the controls. The enhanced properties of the adhesive copolymers indicated that BMPMOB is a promising comonomer for dental restorative materials.
Keywords: crosslink polymer; dentin adhesive; dynamical mechanical property; photopolymerization; water miscibility.
© 2014 Wiley Periodicals, Inc.
Figures




References
-
- Burrow MF, Satoh M, Tagami J. Dentin bond durability after three years using a dentin bonding agent with and without priming. Dent Mater. 1996;12(5–6):302–307. - PubMed
-
- Hashimoto M, Ohno H, Kaga M, Endo K, Sano H, Oguchi H. In vivo degradation of resin-dentin bonds in humans over 1 to 3 years. J Dent Res. 2000;79(6):1385–1391. - PubMed
-
- Hashimoto M, Ohno H, Sano H, Tay FR, Kaga M, Kudou Y, Oguchi H, Araki Y, Kubota M. Micromorphological changes in resin–dentin bonds after 1 year of water storage. J Biomed Mater Res. 2002;63(3):306–311. - PubMed
-
- Sano H, Yoshikawa T, Pereira PNR, Kanemura N, Morigami M, Tagami J, Pashley DH. Long-term durability of dentin bonds made with a self-etching primer, in vivo. J Dent Res. 1999;78(4):906–911. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

