Synaptic vesicle recycling: steps and principles
- PMID: 24596248
- PMCID: PMC4194108
- DOI: 10.1002/embj.201386357
Synaptic vesicle recycling: steps and principles
Abstract
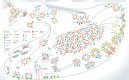
Synaptic vesicle recycling is one of the best-studied cellular pathways. Many of the proteins involved are known, and their interactions are becoming increasingly clear. However, as for many other pathways, it is still difficult to understand synaptic vesicle recycling as a whole. While it is generally possible to point out how synaptic reactions take place, it is not always easy to understand what triggers or controls them. Also, it is often difficult to understand how the availability of the reaction partners is controlled: how the reaction partners manage to find each other in the right place, at the right time. I present here an overview of synaptic vesicle recycling, discussing the mechanisms that trigger different reactions, and those that ensure the availability of reaction partners. A central argument is that synaptic vesicles bind soluble cofactor proteins, with low affinity, and thus control their availability in the synapse, forming a buffer for cofactor proteins. The availability of cofactor proteins, in turn, regulates the different synaptic reactions. Similar mechanisms, in which one of the reaction partners buffers another, may apply to many other processes, from the biogenesis to the degradation of the synaptic vesicle.
Figures



References
-
- Ahmari SE, Buchanan J, Smith SJ. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat Neurosci. 2000;3:445–451. - PubMed
-
- Ahnert-Hilger G, Höltje M, Pahner I, Winter S, Brunk I. Regulation of vesicular neurotransmitter transporters. Rev Physiol Biochem Pharmacol. 2003;150:140–160. - PubMed
-
- Alabi AA, Tsien RW. Perspectives on Kiss-and-Run: role in exocytosis, endocytosis, and neurotransmission. Annu Rev Physiol. 2013;75:393–422. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

