Ethylene-induced flavonol accumulation in guard cells suppresses reactive oxygen species and moderates stomatal aperture
- PMID: 24596331
- PMCID: PMC3982735
- DOI: 10.1104/pp.113.233528
Ethylene-induced flavonol accumulation in guard cells suppresses reactive oxygen species and moderates stomatal aperture
Abstract
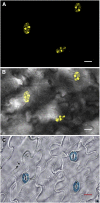
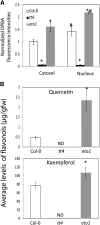
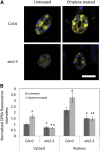
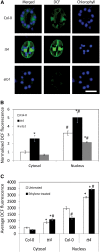
Guard cell swelling controls the aperture of stomata, pores that facilitate gas exchange and water loss from leaves. The hormone abscisic acid (ABA) has a central role in regulation of stomatal closure through synthesis of second messengers, which include reactive oxygen species (ROS). ROS accumulation must be minimized by antioxidants to keep concentrations from reaching damaging levels within the cell. Flavonols are plant metabolites that have been implicated as antioxidants; however, their antioxidant activity in planta has been debated. Flavonols accumulate in guard cells of Arabidopsis thaliana, but not surrounding pavement cells, as visualized with a flavonol-specific dye. The expression of a reporter driven by the promoter of CHALCONE SYNTHASE, a gene encoding a flavonol biosynthetic enzyme, in guard cells, but not pavement cells, suggests guard cell-specific flavonoid synthesis. Increased levels of ROS were detected using a fluorescent ROS sensor in guard cells of transparent testa4-2, which has a null mutation in CHALCONE SYNTHASE and therefore synthesizes no flavonol antioxidants. Guard cells of transparent testa4-2 show more rapid ABA-induced closure than the wild type, suggesting that flavonols may dampen the ABA-dependent ROS burst that drives stomatal closing. The levels of flavonols are positively regulated in guard cells by ethylene treatment in the wild type, but not in the ethylene-insensitive2-5 mutant. In addition, in both ethylene-overproducing1 and ethylene-treated wild-type plants, elevated flavonols lead to decreasing ROS and slower ABA-mediated stomatal closure. These results are consistent with flavonols suppressing ROS accumulation and decreasing the rate of ABA-dependent stomatal closure, with ethylene-induced increases in guard cell flavonols modulating these responses.
Figures




Similar articles
-
Abscisic Acid-Induced Reactive Oxygen Species Are Modulated by Flavonols to Control Stomata Aperture.Plant Physiol. 2017 Dec;175(4):1807-1825. doi: 10.1104/pp.17.01010. Epub 2017 Oct 19. Plant Physiol. 2017. PMID: 29051198 Free PMC article.
-
Ethylene inhibits ABA-induced stomatal closure via regulating NtMYB184-mediated flavonol biosynthesis in tobacco.J Exp Bot. 2023 Nov 21;74(21):6735-6748. doi: 10.1093/jxb/erad308. J Exp Bot. 2023. PMID: 37531314
-
Open Stomata 1 (OST1) is limiting in abscisic acid responses of Arabidopsis guard cells.New Phytol. 2013 Dec;200(4):1049-63. doi: 10.1111/nph.12469. Epub 2013 Sep 3. New Phytol. 2013. PMID: 24033256
-
The Role of ROS Homeostasis in ABA-Induced Guard Cell Signaling.Front Plant Sci. 2020 Jun 30;11:968. doi: 10.3389/fpls.2020.00968. eCollection 2020. Front Plant Sci. 2020. PMID: 32695131 Free PMC article. Review.
-
Mechanism of Stomatal Closure in Plants Exposed to Drought and Cold Stress.Adv Exp Med Biol. 2018;1081:215-232. doi: 10.1007/978-981-13-1244-1_12. Adv Exp Med Biol. 2018. PMID: 30288712 Review.
Cited by
-
Differential regulation of drought stress by biological membrane transporters and channels.Plant Cell Rep. 2021 Aug;40(8):1565-1583. doi: 10.1007/s00299-021-02730-4. Epub 2021 Jun 16. Plant Cell Rep. 2021. PMID: 34132878 Review.
-
Barley Genotypes Vary in Stomatal Responsiveness to Light and CO2 Conditions.Plants (Basel). 2021 Nov 21;10(11):2533. doi: 10.3390/plants10112533. Plants (Basel). 2021. PMID: 34834896 Free PMC article.
-
Abscisic Acid-Induced Reactive Oxygen Species Are Modulated by Flavonols to Control Stomata Aperture.Plant Physiol. 2017 Dec;175(4):1807-1825. doi: 10.1104/pp.17.01010. Epub 2017 Oct 19. Plant Physiol. 2017. PMID: 29051198 Free PMC article.
-
Metabolic Signatures in Response to Abscisic Acid (ABA) Treatment in Brassica napus Guard Cells Revealed by Metabolomics.Sci Rep. 2017 Oct 9;7(1):12875. doi: 10.1038/s41598-017-13166-w. Sci Rep. 2017. PMID: 28993661 Free PMC article.
-
Characterisation of a flavonoid ligand of the fungal protein Alt a 1.Sci Rep. 2016 Sep 16;6:33468. doi: 10.1038/srep33468. Sci Rep. 2016. PMID: 27633190 Free PMC article.
References
-
- Allen GJ, Chu SP, Schumacher K, Shimazaki CT, Vafeados D, Kemper A, Hawke SD, Tallman G, Tsien RY, Harper JF, et al. (2000) Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science 289: 2338–2342 - PubMed
-
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR. (1999) EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284: 2148–2152 - PubMed
-
- Apel K, Hirt H. (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55: 373–399 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

