Global Inhibition of DC Priming Capacity in the Spleen of Self-Antigen Vaccinated Mice Requires IL-10
- PMID: 24596571
- PMCID: PMC3925839
- DOI: 10.3389/fimmu.2014.00059
Global Inhibition of DC Priming Capacity in the Spleen of Self-Antigen Vaccinated Mice Requires IL-10
Abstract
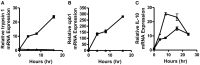
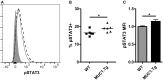
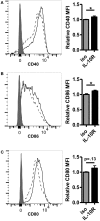
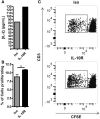
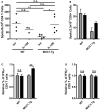
Dendritic cells (DC) in the spleen are highly activated following intravenous vaccination with a foreign-antigen, promoting expansion of effector T cells, but remain phenotypically and functionally immature after vaccination with a self-antigen. Up-regulation or suppression of expression of a cohort of pancreatic enzymes 24-72 h post-vaccination can be used as a biomarker of stimulatory versus tolerogenic DC, respectively. Here we show, using MUC1 transgenic mice and a vaccine based on the MUC1 peptide, which these mice perceive as a self-antigen, that the difference in enzyme expression that predicts whether DC will promote immune response or immune tolerance is seen as early as 4-8 h following vaccination. We also identify early production of IL-10 as a predominant factor that both correlates with this early-time point and controls DC function. Pre-treating mice with an antibody against the IL-10 receptor prior to vaccination results in DC that up-regulate CD40, CD80, and CD86 and promote stronger IFNγ+ T cell responses. This study suggests that transient inhibition of IL-10 prior to vaccination could improve responses to cancer vaccines that utilize self-tumor antigens.
Keywords: IL-10; MUC1; T cell response; cancer vaccine; dendritic cells.
Figures
References
-
- Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol (1997) 159:4772–80 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

