Architecture and function of metallopeptidase catalytic domains
- PMID: 24596965
- PMCID: PMC3926739
- DOI: 10.1002/pro.2400
Architecture and function of metallopeptidase catalytic domains
Abstract
The cleavage of peptide bonds by metallopeptidases (MPs) is essential for life. These ubiquitous enzymes participate in all major physiological processes, and so their deregulation leads to diseases ranging from cancer and metastasis, inflammation, and microbial infection to neurological insults and cardiovascular disorders. MPs cleave their substrates without a covalent intermediate in a single-step reaction involving a solvent molecule, a general base/acid, and a mono- or dinuclear catalytic metal site. Most monometallic MPs comprise a short metal-binding motif (HEXXH), which includes two metal-binding histidines and a general base/acid glutamate, and they are grouped into the zincin tribe of MPs. The latter divides mainly into the gluzincin and metzincin clans. Metzincins consist of globular ∼ 130-270-residue catalytic domains, which are usually preceded by N-terminal pro-segments, typically required for folding and latency maintenance. The catalytic domains are often followed by C-terminal domains for substrate recognition and other protein-protein interactions, anchoring to membranes, oligomerization, and compartmentalization. Metzincin catalytic domains consist of a structurally conserved N-terminal subdomain spanning a five-stranded β-sheet, a backing helix, and an active-site helix. The latter contains most of the metal-binding motif, which is here characteristically extended to HEXXHXXGXX(H,D). Downstream C-terminal subdomains are generally shorter, differ more among metzincins, and mainly share a conserved loop--the Met-turn--and a C-terminal helix. The accumulated structural data from more than 300 deposited structures of the 12 currently characterized metzincin families reviewed here provide detailed knowledge of the molecular features of their catalytic domains, help in our understanding of their working mechanisms, and form the basis for the design of novel drugs.
Figures



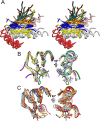
Similar articles
-
Structural aspects of the metzincin clan of metalloendopeptidases.Mol Biotechnol. 2003 Jun;24(2):157-202. doi: 10.1385/MB:24:2:157. Mol Biotechnol. 2003. PMID: 12746556 Review.
-
Catalytic domain architecture of metzincin metalloproteases.J Biol Chem. 2009 Jun 5;284(23):15353-7. doi: 10.1074/jbc.R800069200. Epub 2009 Feb 5. J Biol Chem. 2009. PMID: 19201757 Free PMC article.
-
A standard orientation for metallopeptidases.Biochim Biophys Acta. 2012 Jan;1824(1):157-63. doi: 10.1016/j.bbapap.2011.04.014. Epub 2011 Apr 30. Biochim Biophys Acta. 2012. PMID: 21558023 Review.
-
Matrix metalloproteinases: fold and function of their catalytic domains.Biochim Biophys Acta. 2010 Jan;1803(1):20-8. doi: 10.1016/j.bbamcr.2009.04.003. Epub 2009 Apr 15. Biochim Biophys Acta. 2010. PMID: 19374923 Review.
-
A novel family of soluble minimal scaffolds provides structural insight into the catalytic domains of integral membrane metallopeptidases.J Biol Chem. 2013 Jul 19;288(29):21279-21294. doi: 10.1074/jbc.M113.476580. Epub 2013 Jun 3. J Biol Chem. 2013. PMID: 23733187 Free PMC article.
Cited by
-
Stanniocalcin-2 inhibits mammalian growth by proteolytic inhibition of the insulin-like growth factor axis.J Biol Chem. 2015 Feb 6;290(6):3430-9. doi: 10.1074/jbc.M114.611665. Epub 2014 Dec 22. J Biol Chem. 2015. PMID: 25533459 Free PMC article.
-
DPP3 expression promotes cell proliferation and migration in vitro and tumour growth in vivo, which is associated with poor prognosis of oesophageal carcinoma.Oncol Rep. 2023 Jan;49(1):9. doi: 10.3892/or.2022.8446. Epub 2022 Nov 16. Oncol Rep. 2023. PMID: 36382663 Free PMC article.
-
A conserved signaling pathway activates bacterial CBASS immune signaling in response to DNA damage.EMBO J. 2022 Nov 17;41(22):e111540. doi: 10.15252/embj.2022111540. Epub 2022 Sep 26. EMBO J. 2022. PMID: 36156805 Free PMC article.
-
A cryptic third active site in cyanophycin synthetase creates primers for polymerization.Nat Commun. 2022 Jul 7;13(1):3923. doi: 10.1038/s41467-022-31542-7. Nat Commun. 2022. PMID: 35798723 Free PMC article.
-
Plectreurys tristis venome: A proteomic and transcriptomic analysis.J Venom Res. 2014 Sep 20;5:33-47. eCollection 2014. J Venom Res. 2014. PMID: 25400903 Free PMC article.
References
-
- Barrett AJ, Rawlings ND, Salvesen G, Woessner JF. In: Handbook of proteolytic enzymes. Rawlings ND, Salvesen G, editors. Oxford (UK): Academic Press; 2013. pp. Ii–Iiv.
-
- Neurath H. Limited proteolysis and zymogen activation. In: Reich E, Rifkins DB, Shaw E, editors. Proteases and biological control. New York: Cold Spring Harbor Laboratory Press; 1975. pp. 51–64.
-
- López-Otín C, Overall CM. Protease degradomics: a new challenge for proteomics. Nat Rev Mol Cell Biol. 2002;3:509–519. - PubMed
-
- Kheradmand F, Werb Z. Shedding light on sheddases: role in growth and development. BioEssays. 2002;24:8–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

