Synthetic FXR agonist GW4064 is a modulator of multiple G protein-coupled receptors
- PMID: 24597548
- PMCID: PMC5414852
- DOI: 10.1210/me.2013-1353
Synthetic FXR agonist GW4064 is a modulator of multiple G protein-coupled receptors
Abstract
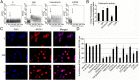
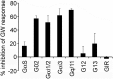
The synthetic nuclear bile acid receptor (farnesoid X receptor [FXR]) agonist GW4064 is extensively used as a specific pharmacological tool to illustrate FXR functions. We noticed that GW4064 activated empty luciferase reporters in FXR-deficient HEK-293T cells. We postulated that this activity of GW4064 might be routed through as yet unknown cellular targets and undertook an unbiased exploratory approach to identify these targets. Investigations revealed that GW4064 activated cAMP and nuclear factor for activated T-cell response elements (CRE and NFAT-RE, respectively) present on these empty reporters. Whereas GW4064-induced NFAT-RE activation involved rapid intracellular Ca(2+) accumulation and NFAT nuclear translocation, CRE activation involved soluble adenylyl cyclase-dependent cAMP accumulation and Ca(2+)-calcineurin-dependent nuclear translocation of transducers of regulated CRE-binding protein 2. Use of dominant negative heterotrimeric G-protein minigenes revealed that GW4064 caused activation of Gαi/o and Gq/11 G proteins. Sequential pharmacological inhibitor-based screening and radioligand-binding studies revealed that GW4064 interacted with multiple G protein-coupled receptors. Functional studies demonstrated that GW4064 robustly activated H1 and H4 and inhibited H2 histamine receptor signaling events. We also found that MCF-7 breast cancer cells, reported to undergo GW4064-induced apoptosis in an FXR-dependent manner, did not express FXR, and the GW4064-mediated apoptosis, also apparent in HEK-293T cells, could be blocked by selective histamine receptor regulators. Taken together, our results demonstrate identification of histamine receptors as alternate targets for GW4064, which not only necessitates cautious interpretation of the biological functions attributed to FXR using GW4064 as a pharmacological tool but also provides a basis for the rational designing of new pharmacophores for histamine receptor modulation.
Figures





References
-
- Forman BM, Goode E, Chen J, et al. . Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81:687–693. - PubMed
-
- Seol W, Choi HS, Moore DD. Isolation of proteins that interact specifically with the retinoid X receptor: two novel orphan receptors. Mol Endocrinol. 1995;9:72–85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

