Three clinically distinct chronic pediatric airway infections share a common core microbiota
- PMID: 24597615
- PMCID: PMC4214061
- DOI: 10.1513/AnnalsATS.201312-456OC
Three clinically distinct chronic pediatric airway infections share a common core microbiota
Abstract
Rationale: DNA-based microbiological studies are moving beyond studying healthy human microbiota to investigate diverse infectious diseases, including chronic respiratory infections, such as those in the airways of people with cystic fibrosis (CF) and non-CF bronchiectasis. The species identified in the respiratory secretion microbiota from such patients can be classified into those that are common and abundant among similar subjects (core) versus those that are infrequent and rare (satellite). This categorization provides a vital foundation for investigating disease pathogenesis and improving therapy. However, whether the core microbiota of people with different respiratory diseases, which are traditionally associated with specific culturable pathogens, are unique or shared with other chronic infections of the lower airways is not well studied. Little is also known about how these chronic infection microbiota change from childhood to adulthood.
Objectives: We sought to compare the core microbiota in respiratory specimens from children and adults with different chronic lung infections.
Methods: We used bacterial 16S rRNA gene pyrosequencing, phylogenetic analysis, and ecological statistical tools to compare the core microbiota in respiratory samples from three cohorts of symptomatic children with clinically distinct airway diseases (protracted bacterial bronchitis, bronchiectasis, CF), and from four healthy children. We then compared the core pediatric respiratory microbiota with those in samples from adults with bronchiectasis and CF.
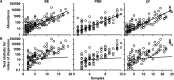
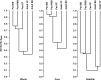
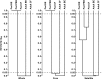
Measurements and main results: All three pediatric disease cohorts shared strikingly similar core respiratory microbiota that differed from adult CF and bronchiectasis microbiota. The most common species in pediatric disease cohort samples were also detected in those from healthy children. The adult CF and bronchiectasis microbiota also differed from each other, suggesting common early infection airway microbiota that diverge by adulthood. The shared core pediatric microbiota included both traditional pathogens and many species not routinely identified by standard culture.
Conclusions: Our results indicate that these clinically distinct chronic airway infections share common early core microbiota, which are likely shaped by natural aspiration and impaired clearance of the same airway microbes, but that disease-specific characteristics select for divergent microbiota by adulthood. Longitudinal and interventional studies will be required to define the relationships between microbiota, treatments, and disease progression.
Keywords: bronchiectasis; core microbiota; cystic fibrosis; protracted bacterial bronchitis.
Figures


Comment in
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

