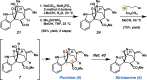
Total synthesis of the akuammiline alkaloid picrinine
- PMID: 24597784
- PMCID: PMC3985766
- DOI: 10.1021/ja501780w
Total synthesis of the akuammiline alkaloid picrinine
Abstract
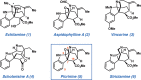
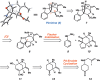
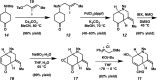
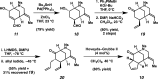
We report the first total synthesis of the complex akuammiline alkaloid picrinine, which was first isolated nearly five decades ago. Our synthetic approach features a concise assembly of the [3.3.1]-azabicyclic core, a key Fischer indolization reaction to forge the natural product's carbon framework, and a series of delicate late-stage transformations to complete the synthesis. Our synthesis of picrinine also constitutes a formal synthesis of the related polycyclic alkaloid strictamine.
Figures
References
-
-
For reviews on akuammiline alkaloids, see:
- Ramírez A.; García–Rubio S. Curr. Med. Chem. 2003, 10, 1891–1915. - PubMed
- Eckermann R.; Gaich T. Synthesis 2013, 45, 2813–2823.
-
-
- For the initial isolation of 1, see:Group-Besanez v. Justus Liebigs Ann. Chem. 1875, 176, 88–89.
-
- For the isolation of 2, see:Subramaniam G.; Hiraku O.; Hayashi M.; Koyano T.; Komiyama K.; Kam T.-S. J. Nat. Prod. 2007, 70, 1783–1789. - PubMed
-
- For the initial isolation of 3, see:Mokŕý J.; Dúbravková L.; Šefčovič P. Experientia 1962, 18, 564–565. - PubMed
-
- For the isolation of 4, see:Cai X.-H.; Tan Q.-G.; Liu Y.-P.; Feng T.; Du Z.-Z.; Li W.-Q.; Luo X.-D. Org. Lett. 2008, 10, 577–580. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources