Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV
- PMID: 24597865
- PMCID: PMC4084652
- DOI: 10.1056/NEJMoa1300662
Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV
Abstract
Background: CCR5 is the major coreceptor for human immunodeficiency virus (HIV). We investigated whether site-specific modification of the gene ("gene editing")--in this case, the infusion of autologous CD4 T cells in which the CCR5 gene was rendered permanently dysfunctional by a zinc-finger nuclease (ZFN)--is safe.
Methods: We enrolled 12 patients in an open-label, nonrandomized, uncontrolled study of a single dose of ZFN-modified autologous CD4 T cells. The patients had chronic aviremic HIV infection while they were receiving highly active antiretroviral therapy. Six of them underwent an interruption in antiretroviral treatment 4 weeks after the infusion of 10 billion autologous CD4 T cells, 11 to 28% of which were genetically modified with the ZFN. The primary outcome was safety as assessed by treatment-related adverse events. Secondary outcomes included measures of immune reconstitution and HIV resistance.
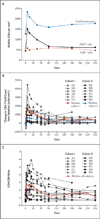
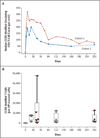
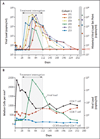
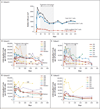
Results: One serious adverse event was associated with infusion of the ZFN-modified autologous CD4 T cells and was attributed to a transfusion reaction. The median CD4 T-cell count was 1517 per cubic millimeter at week 1, a significant increase from the preinfusion count of 448 per cubic millimeter (P<0.001). The median concentration of CCR5-modified CD4 T cells at 1 week was 250 cells per cubic millimeter. This constituted 8.8% of circulating peripheral-blood mononuclear cells and 13.9% of circulating CD4 T cells. Modified cells had an estimated mean half-life of 48 weeks. During treatment interruption and the resultant viremia, the decline in circulating CCR5-modified cells (-1.81 cells per day) was significantly less than the decline in unmodified cells (-7.25 cells per day) (P=0.02). HIV RNA became undetectable in one of four patients who could be evaluated. The blood level of HIV DNA decreased in most patients.
Conclusions: CCR5-modified autologous CD4 T-cell infusions are safe within the limits of this study. (Funded by the National Institute of Allergy and Infectious Diseases and others; ClinicalTrials.gov number, NCT00842634.).
Figures
Comment in
-
Engineering cellular resistance to HIV.N Engl J Med. 2014 Mar 6;370(10):968-9. doi: 10.1056/NEJMe1400593. N Engl J Med. 2014. PMID: 24597871 No abstract available.
-
[Gene therapy in HIV infection: proof-of-concept in 12 patients].Med Mal Infect. 2014 May;44(5):239-40. doi: 10.1016/j.medmal.2014.03.011. Med Mal Infect. 2014. PMID: 25035879 French. No abstract available.
References
-
- Igoucheva O, Alexeev V, Yoon K. Oligo-nucleotide-directed mutagenesis and targeted gene correction: a mechanistic point of view. Curr Mol Med. 2004;4:445–463. - PubMed
-
- Kim KH, Nielsen PE, Glazer PM. Site-specific gene modification by PNAs conjugated to psoralen. Biochemistry. 2006;45:314–323. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
