Enzymatic Characterization of ER Stress-Dependent Kinase, PERK, and Development of a High-Throughput Assay for Identification of PERK Inhibitors
- PMID: 24598103
- PMCID: PMC4570879
- DOI: 10.1177/1087057114525853
Enzymatic Characterization of ER Stress-Dependent Kinase, PERK, and Development of a High-Throughput Assay for Identification of PERK Inhibitors
Abstract
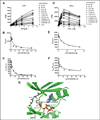
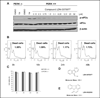
PERK is serine/threonine kinase localized to the endoplasmic reticulum (ER) membrane. PERK is activated and contributes to cell survival in response to a variety of physiological stresses that affect protein quality control in the ER, such as hypoxia, glucose depravation, increased lipid biosynthesis, and increased protein translation. Pro-survival functions of PERK are triggered by such stresses, suggesting that development of small-molecule inhibitors of PERK may be efficacious in a variety of disease scenarios. Hence, we have conducted a detailed enzymatic characterization of the PERK kinase to develop a high-throughput-screening assay (HTS) that will permit the identification of small-molecule PERK inhibitors. In addition to establishing the K(m) of PERK for both its primary substrate, eIF2α, and for adenosine triphosphate, further mechanistic studies revealed that PERK targets its substrate via either a random/steady-state ordered mechanism. For HTS, we developed a time-resolved fluorescence resonance energy transfer-based assay that yielded a robust Z' factor and percent coefficient of variation value, enabling the successful screening of 79,552 compounds. This approach yielded one compound that exhibited good in vitro and cellular activity. These results demonstrate the validity of this screen and represent starting points for drug discovery efforts.
Keywords: HTS; PERK; small-molecule inhibitors.
© 2014 Society for Laboratory Automation and Screening.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures




References
-
- Yamamoto K, Sato T, Matsui T, et al. Transcriptional Induction of Mammalian ER Quality Control Proteins Is Mediated by Single or Combined Action of ATF6alpha and XBP1. Dev. Cell. 2007;13:365–376. - PubMed
-
- Wu J, Rutkowski DT, Dubois M, et al. ATF6alpha Optimizes Long-Term Endoplasmic Reticulum Function to Protect Cells from Chronic Stress. Dev. Cell. 2007;13:351–364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

