Longitudinal change in CSF biomarkers in autosomal-dominant Alzheimer's disease
- PMID: 24598588
- PMCID: PMC4038930
- DOI: 10.1126/scitranslmed.3007901
Longitudinal change in CSF biomarkers in autosomal-dominant Alzheimer's disease
Abstract
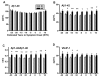
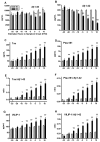
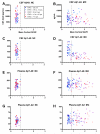
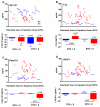
Clinicopathological evidence suggests that the pathology of Alzheimer's disease (AD) begins many years before the appearance of cognitive symptoms. Biomarkers are required to identify affected individuals during this asymptomatic ("preclinical") stage to permit intervention with potential disease-modifying therapies designed to preserve normal brain function. Studies of families with autosomal-dominant AD (ADAD) mutations provide a unique and powerful means to investigate AD biomarker changes during the asymptomatic period. In this biomarker study, we collected cerebrospinal fluid (CSF), plasma, and in vivo amyloid imaging cross-sectional data at baseline in individuals from ADAD families enrolled in the Dominantly Inherited Alzheimer Network. Our study revealed reduced concentrations of CSF amyloid-β1-42 (Aβ1-42) associated with the presence of Aβ plaques, and elevated concentrations of CSF tau, ptau181 (phosphorylated tau181), and VILIP-1 (visinin-like protein-1), markers of neurofibrillary tangles and neuronal injury/death, in asymptomatic mutation carriers 10 to 20 years before their estimated age at symptom onset (EAO) and before the detection of cognitive deficits. When compared longitudinally, however, the concentrations of CSF biomarkers of neuronal injury/death within individuals decreased after their EAO, suggesting a slowing of acute neurodegenerative processes with symptomatic disease progression. These results emphasize the importance of longitudinal, within-person assessment when modeling biomarker trajectories across the course of the disease. If corroborated, this pattern may influence the definition of a positive neurodegenerative biomarker outcome in clinical trials.
Figures

References
-
- Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–357. - PubMed
-
- Hulette CM, Welsh-Bohmer KA, Murray MG, Saunders AM, Mash DC, McIntyre LM. Neuropathological and neuropsychological changes in “normal” aging: Evidence for preclinical Alzheimer disease in cognitively normal individuals. J Neuropathol Exp Neurol. 1998;57:1168–1174. - PubMed
-
- Markesbery W, Schmitt F, Kryscio R, Davis D, Smith C, Wekstein D. Neuropathologic substrate of Mild Cognitive Impairment. Arch Neurol. 2006;63:38–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 NS057105/NS/NINDS NIH HHS/United States
- U01 AG032438/AG/NIA NIH HHS/United States
- K24 AG035007/AG/NIA NIH HHS/United States
- U24 AG021886/AG/NIA NIH HHS/United States
- P01 AG036694/AG/NIA NIH HHS/United States
- P01 AG026276/AG/NIA NIH HHS/United States
- P30 NS048056/NS/NINDS NIH HHS/United States
- UF1 AG032438/AG/NIA NIH HHS/United States
- P50AG016750/AG/NIA NIH HHS/United States
- U19AG032438/AG/NIA NIH HHS/United States
- U19 AG032438/AG/NIA NIH HHS/United States
- P01 AG003991/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- R01 EB009352/EB/NIBIB NIH HHS/United States
- P30 NS098577/NS/NINDS NIH HHS/United States
- P50 AG005134/AG/NIA NIH HHS/United States
- P30 AR048335/AR/NIAMS NIH HHS/United States
- R01 AG016750/AG/NIA NIH HHS/United States
- KL2 RR024994/RR/NCRR NIH HHS/United States
- U24 AG21886/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

