Preferential macrophage recruitment and polarization in LPS-induced animal model for COPD: noninvasive tracking using MRI
- PMID: 24598763
- PMCID: PMC3945006
- DOI: 10.1371/journal.pone.0090829
Preferential macrophage recruitment and polarization in LPS-induced animal model for COPD: noninvasive tracking using MRI
Abstract
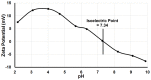
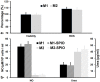
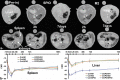
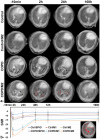
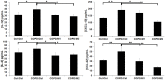
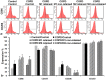
Noninvasive imaging of macrophages activity has raised increasing interest for diagnosis of chronic obstructive respiratory diseases (COPD), which make them attractive vehicles to deliver contrast agents for diagnostic or drugs for therapeutic purposes. This study was designed to monitor and evaluate the migration of differently polarized M1 and M2 iron labeled macrophage subsets to the lung of a LPS-induced COPD animal model and to assess their polarization state once they have reached the inflammatory sites in the lung after intravenous injection. Ex vivo polarized bone marrow derived M1 or M2 macrophages were first efficiently and safely labeled with amine-modified PEGylated dextran-coated SPIO nanoparticles and without altering their polarization profile. Their biodistribution in abdominal organs and their homing to the site of inflammation in the lung was tracked for the first time using a free-breathing non-invasive MR imaging protocol on a 4.7T magnet after their intravenous administration. This imaging protocol was optimized to allow both detection of iron labeled macrophages and visualization of inflammation in the lung. M1 and M2 macrophages were successfully detected in the lung starting from 2 hours post injection with no variation in their migration profile. Quantification of cytokines release, analysis of surface membrane expression using flow cytometry and immunohistochemistry investigations confirmed the successful recruitment of injected iron labeled macrophages in the lung of COPD mice and revealed that even with a continuum switch in the polarization profile of M1 and M2 macrophages during the time course of inflammation a balanced number of macrophage subsets predominate.
Conflict of interest statement
Figures

References
-
- Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. Global Initiative for Obstructive Lung Disease. Available: http://www.goldcopd.com. Accessed 2013 Feb 20: GOLD 2013.
-
- Barnes P (2004) Alveolar Macrophages as Orchestrators of COPD. COPD: Journal of Chronic Obstructive Pulmonary Disease 1: 59–70. - PubMed
-
- Biswas SK, Chittezhath M, Shalova IN, Lim JY (2012) Macrophage polarization and plasticity in health and disease. Immunol Res 53: 11–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

