The regulation of spermatogenesis by androgens
- PMID: 24598768
- PMCID: PMC4043871
- DOI: 10.1016/j.semcdb.2014.02.012
The regulation of spermatogenesis by androgens
Abstract
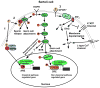
Testosterone is essential for maintaining spermatogenesis and male fertility. However, the molecular mechanisms by which testosterone acts have not begun to be revealed until recently. With the advances obtained from the use of transgenic mice lacking or overexpressing the androgen receptor, the cell specific targets of testosterone action as well as the genes and signaling pathways that are regulated by testosterone are being identified. In this review, the critical steps of spermatogenesis that are regulated by testosterone are discussed as well as the intracellular signaling pathways by which testosterone acts. We also review the functional information that has been obtained from the knock out of the androgen receptor from specific cell types in the testis and the genes found to be regulated after altering testosterone levels or androgen receptor expression.
Keywords: Blood testis barrier; Fertility; Meiosis; Sertoli cell; Testis; Testosterone.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

