p-Hydroxyphenylpyruvate, an intermediate of the Phe/Tyr catabolism, improves mitochondrial oxidative metabolism under stressing conditions and prolongs survival in rats subjected to profound hemorrhagic shock
- PMID: 24599095
- PMCID: PMC3944966
- DOI: 10.1371/journal.pone.0090917
p-Hydroxyphenylpyruvate, an intermediate of the Phe/Tyr catabolism, improves mitochondrial oxidative metabolism under stressing conditions and prolongs survival in rats subjected to profound hemorrhagic shock
Abstract
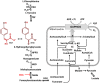
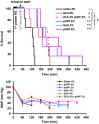
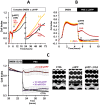
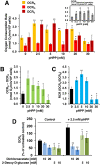
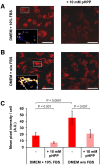
The aim of this study was to test the effect of a small volume administration of p-hydroxyphenylpyruvate (pHPP) in a rat model of profound hemorrhagic shock and to assess a possible metabolic mechanism of action of the compound. The results obtained show that hemorrhaged rats treated with 2-4% of the estimated blood volume of pHPP survived significantly longer (p<0.001) than rats treated with vehicle. In vitro analysis on cultured EA.hy 926 cells demonstrated that pHPP improved cell growth rate and promoted cell survival under stressing conditions. Moreover, pHPP stimulated mitochondria-related respiration under ATP-synthesizing conditions and exhibited antioxidant activity toward mitochondria-generated reactive oxygen species. The compound effects reported in the in vitro and in vivo analyses were obtained in the same millimolar concentration range. These data disclose pHPP as an efficient energetic substrates-supplier to the mitochondrial respiratory chain as well as an antioxidant supporting the view that the compound warrants further evaluation as a therapeutic agent.
Conflict of interest statement
Figures

References
-
- Kauvar DS, Lefering R, Wade CE (2006) Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma 60(6 Suppl): S3–11. - PubMed
-
- Peitzman AB, Billiar TR, Harbrecht BG, Kelly E, Udekwu AO, et al. (1995) Hemorrhagic shock. Curr Probl Surg 32: 925–1002. - PubMed
-
- Cairns CB (2001) Rude unhinging of the machinery of life: metabolic approaches to hemorrhagic shock. Opin Crit Care 7: 437–443. - PubMed
-
- McSwain NE, Champion HR, Fabian TC, Hoyt DB, Wade CE, et al.. (2010) State of the art of fluid resuscitation 2010: prehospital and immediate transition to the hospital. J Trauma 70(5 Suppl): S2–10. - PubMed
-
- Riddez L, Drobin D, Sjostrand F, Svensen C, Hahn RG (2002) Lower dose of hypertonic saline dextran reduces the risk of lethal rebleeding in uncontrolled hemorrhage. Shock 17: 377–382. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

