Effects of blood contamination and the rostro-caudal gradient on the human cerebrospinal fluid proteome
- PMID: 24599184
- PMCID: PMC3943968
- DOI: 10.1371/journal.pone.0090429
Effects of blood contamination and the rostro-caudal gradient on the human cerebrospinal fluid proteome
Abstract
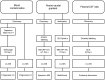
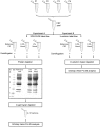
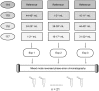
Over the last years there has been an increased focus on the importance of knowing the effect of pre-analytical influence on the proteomes under study, particularly in the field of biomarker discovery. We present three proteomics studies examining the effect of blood contamination and the rostro-caudal gradient (RCG) on the cerebrospinal fluid (CSF) proteome, in addition to plasma/CSF protein ratios. The studies showed that the central nervous system (CNS) derived proteins appeared to be unaffected by the RCG, while the plasma-derived proteins showed an increase in concentration towards the lumbar area. This implies that the concentration of the plasma-derived proteins in CSF will vary depending on the volume of CSF that is collected. In the CSF samples spiked with blood, 262 of 814 quantified proteins showed an abundance increase of more than 1.5 fold, while 403 proteins had a fold change of less than 1.2 and appeared to be unaffected by blood contamination. Proteins with a high plasma/CSF ratio appeared to give the largest effect on the CSF proteome upon blood contamination. The results give important background information on how factors like blood contamination, RCG and blood-CNS-barrier influences the CSF proteome. This information is particularly important in the field of biomarker discovery, but also for routine clinical measurements. The data from the blood contamination and RCG discovery studies have been deposited to the ProteomeXchange with identifier PXD000401.
Conflict of interest statement
Figures


References
-
- Simonsen AH, Bahl JM, Danborg PB, Lindstrom V, Larsen SO, et al. (2013) Pre-analytical factors influencing the stability of cerebrospinal fluid proteins. J Neurosci Methods 215: 234–240. - PubMed
-
- Berven FS, Kroksveen AC, Berle M, Rajalahti T, Flikka K, et al. (2007) Pre-analytical influence on the low molecular weight cerebrospinal fluid proteome. Proteomics Clinical Applications 1: 699–711. - PubMed
-
- Jimenez CR, Koel-Simmelink M, Pham TV, van der Voort L, Teunissen CE (2007) Endogeneous peptide profiling of cerebrospinal fluid by MALDI-TOF mass spectrometry: Optimization of magnetic bead-based peptide capture and analysis of preanalytical variables. Proteomics Clin Appl 1: 1385–1392. - PubMed
-
- Petzold A, Sharpe LT, Keir G (2006) Spectrophotometry for cerebrospinal fluid pigment analysis. Neurocrit Care 4: 153–162. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

