Staphylococcal bicomponent pore-forming toxins: targets for prophylaxis and immunotherapy
- PMID: 24599233
- PMCID: PMC3968370
- DOI: 10.3390/toxins6030950
Staphylococcal bicomponent pore-forming toxins: targets for prophylaxis and immunotherapy
Abstract
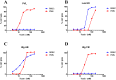
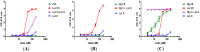
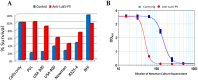
Staphylococccus aureus represents one of the most challenging human pathogens as well as a common colonizer of human skin and mucosal surfaces. S. aureus causes a wide range of diseases from skin and soft tissue infection (SSTI) to debilitating and life-threatening conditions such as osteomyelitis, endocarditis, and necrotizing pneumonia. The range of diseases reflects the remarkable diversity of the virulence factors produced by this pathogen, including surface antigens involved in the establishment of infection and a large number of toxins that mediate a vast array of cellular responses. The staphylococcal toxins are generally believed to have evolved to disarm the innate immune system, the first line of defense against this pathogen. This review focuses on recent advances on elucidating the biological functions of S. aureus bicomponent pore-forming toxins (BCPFTs) and their utility as targets for preventive and therapeutic intervention. These toxins are cytolytic to a variety of immune cells, primarily neutrophils, as well as cells with a critical barrier function. The lytic activity of BCPFTs towards immune cells implies a critical role in immune evasion, and a number of epidemiological studies and animal experiments relate these toxins to clinical disease, particularly SSTI and necrotizing pneumonia. Antibody-mediated neutralization of this lytic activity may provide a strategy for development of toxoid-based vaccines or immunotherapeutics for prevention or mitigation of clinical diseases. However, certain BCPFTs have been proposed to act as danger signals that may alert the immune system through an inflammatory response. The utility of a neutralizing vaccination strategy must be weighed against such immune-activating potential.
Figures
References
-
- Tristan A., Ferry T., Durand G., Dauwalder O., Bes M., Lina G., Vandenesch F., Etienne J. Virulence determinants in community and hospital meticillin-resistant staphylococcus aureus. J. Hosp. Infect. 2007;65(Suppl. 2):105–109. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

