Differential neuroprotective effects of 5'-deoxy-5'-methylthioadenosine
- PMID: 24599318
- PMCID: PMC3944389
- DOI: 10.1371/journal.pone.0090671
Differential neuroprotective effects of 5'-deoxy-5'-methylthioadenosine
Abstract
Background: 5'-deoxy-5'-methylthioadenosine (MTA) is an endogenous compound produced through the metabolism of polyamines. The therapeutic potential of MTA has been assayed mainly in liver diseases and, more recently, in animal models of multiple sclerosis. The aim of this study was to determine the neuroprotective effect of this molecule in vitro and to assess whether MTA can cross the blood brain barrier (BBB) in order to also analyze its potential neuroprotective efficacy in vivo.
Methods: Neuroprotection was assessed in vitro using models of excitotoxicity in primary neurons, mixed astrocyte-neuron and primary oligodendrocyte cultures. The capacity of MTA to cross the BBB was measured in an artificial membrane assay and using an in vitro cell model. Finally, in vivo tests were performed in models of hypoxic brain damage, Parkinson's disease and epilepsy.
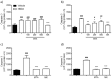
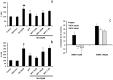
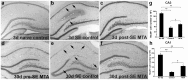
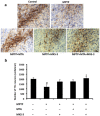
Results: MTA displays a wide array of neuroprotective activities against different insults in vitro. While the data from the two complementary approaches adopted indicate that MTA is likely to cross the BBB, the in vivo data showed that MTA may provide therapeutic benefits in specific circumstances. Whereas MTA reduced the neuronal cell death in pilocarpine-induced status epilepticus and the size of the lesion in global but not focal ischemic brain damage, it was ineffective in preserving dopaminergic neurons of the substantia nigra in the 1-methyl-4-phenyl-1,2,3,6-tetrahydro-pyridine (MPTP)-mice model. However, in this model of Parkinson's disease the combined administration of MTA and an A2A adenosine receptor antagonist did produce significant neuroprotection in this brain region.
Conclusion: MTA may potentially offer therapeutic neuroprotection.
Conflict of interest statement
Figures

References
-
- Williams-Ashman HG, Seidenfeld J, Galletti P (1982) Trends in the biochemical pharmacology of 5′-deoxy-5′-methylthioadenosine. Biochem Pharmacol 31(3): 277–88. - PubMed
-
- Avila MA, García-Trevijano ER, Lu SC, Corrales FJ, Mato JM (2004) Methylthioadenosine. Int J Biochem Cell Biol 36(11): 2125–30. - PubMed
-
- Latasa MU, Gil-Puig C, Fernández-Barrena MG, Rodríguez-Ortigosa CM, Banales JM, et al. (2010) Oral Methylthioadenosine Administration Attenuates Fibrosis and Chronic Liver Disease Progression in Mdr22/2 Mice. PLoS ONE 5(12): e15690 doi:10.1371/journal.pone.0015690 - DOI - PMC - PubMed
-
- Moreno B, Hevia H, Santamaria M, Sepulcre J, Muñoz J, et al. (2006) Methylthioadenosine reverses brain autoimmune disease. Ann Neurol 60(3): 323–34. - PubMed
-
- Moreno B, Fernandez-Diez B, Di Penta A, Villoslada P (2010) Preclinical studies of methylthioadenosine for the treatment of multiple sclerosis. Mult Scler 16(9): 1102–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

