Prenatal deletion of the RNA-binding protein HuD disrupts postnatal cortical circuit maturation and behavior
- PMID: 24599466
- PMCID: PMC3942583
- DOI: 10.1523/JNEUROSCI.3703-13.2014
Prenatal deletion of the RNA-binding protein HuD disrupts postnatal cortical circuit maturation and behavior
Abstract
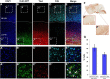
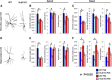
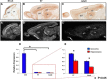
The proper functions of cortical circuits are dependent upon both appropriate neuronal subtype specification and their maturation to receive appropriate signaling. These events establish a balanced circuit that is important for learning, memory, emotion, and complex motor behaviors. Recent research points to mRNA metabolism as a key regulator of this development and maturation process. Hu antigen D (HuD), an RNA-binding protein, has been implicated in the establishment of neuronal identity and neurite outgrowth in vitro. Therefore, we investigated the role of HuD loss of function on neuron specification and dendritogenesis in vivo using a mouse model. We found that loss of HuD early in development results in a defective early dendritic overgrowth phase and pervasive deficits in neuron specification in the lower neocortical layers and defects in dendritogenesis in the CA3 region of the hippocampus. Subsequent behavioral analysis revealed a deficit in performance of a hippocampus-dependent task: the Morris water maze. Further, HuD knock-out (KO) mice exhibited lower levels of anxiety than their wild-type counterparts and were overall less active. Last, we found that HuD KO mice are more susceptible to auditory-induced seizures, often resulting in death. Our findings suggest that HuD is necessary for the establishment of neocortical and hippocampal circuitry and is critical for their function.
Figures






References
-
- Akamatsu W, Fujihara H, Mitsuhashi T, Yano M, Shibata S, Hayakawa Y, Okano HJ, Sakakibara S, Takano H, Takano T, Takahashi T, Noda T, Okano H. The RNA-binding protein HuD regulates neuronal cell identity and maturation. Proc Natl Acad Sci U S A. 2005;102:4625–4630. doi: 10.1073/pnas.0407523102. - DOI - PMC - PubMed
-
- Anderson KD, Morin MA, Beckel-Mitchener A, Mobarak CD, Neve RL, Furneaux HM, Burry R, Perrone-Bizzozero NI. Overexpression of HuD, but not of its truncated form HuD I+II, promotes GAP-43 gene expression and neurite outgrowth in PC12 cells in the absence of nerve growth factor. J Neurochem. 2000;75:1103–1114. doi: 10.1046/j.1471-4159.2000.0751103.x. - DOI - PubMed
-
- Anderson KD, Sengupta J, Morin M, Neve RL, Valenzuela CF, Perrone-Bizzozero NI. Overexpression of HuD accelerates neurite outgrowth and increases GAP-43 mRNA expression in cortical neurons and retinoic acid-induced embryonic stem cells in vitro. Exp Neurol. 2001;168:250–258. doi: 10.1006/exnr.2000.7599. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
