Chaperone potential of Pulicaria undulata extract in preventing aggregation of stressed proteins
- PMID: 24599512
- PMCID: PMC4037491
- DOI: 10.1208/s12249-014-0090-2
Chaperone potential of Pulicaria undulata extract in preventing aggregation of stressed proteins
Abstract
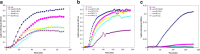
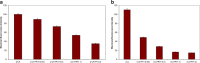
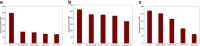
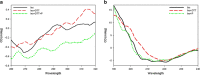
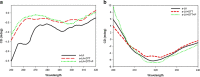
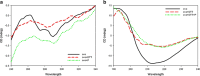
This study examined the effect of an aqueous extract of Pulicaria undulata on the 1,4-dithiothreitol (DTT)-induced aggregation of proteins. The effects of the chaperone properties of P. undulata extract on protein aggregation were determined by measuring light scattering absorption, fluorescence, and circular dichroism (CD) spectroscopy. The aqueous extract of P. undulata possesses good chaperone properties but the protection effect was varied in different protein. The extract showed a higher level of protection in high molecular weight proteins than in those of low molecular weight. Using a fluorescence study, the present study provides information on the hydrophobic area of proteins interacting with the P. undulata extract. In fact, by increasing the concentration of the P. undulata extract, the hydrophic area of the protein decreased. CD spectroscopy also revealed that DTT caused changes in both the tertiary and the secondary structure of the proteins, while in the presence of P. undulata extract, there was little change. Our finding suggests the possibility of using P. undulata extract for the inhibition of aggregation and the deposition of protein in disease.
Figures
Similar articles
-
The effect of green synthesis silver nanoparticles (AgNPs) from Pulicaria undulata on the amyloid formation in α-lactalbumin and the chaperon action of α-casein.Int J Biol Macromol. 2018 Mar;108:1128-1139. doi: 10.1016/j.ijbiomac.2017.12.040. Epub 2017 Dec 7. Int J Biol Macromol. 2018. PMID: 29225181
-
Assessment of anticancer activity of Pulicaria undulata on hepatocellular carcinoma HepG2 cell line.Tumour Biol. 2019 Oct;41(10):1010428319880080. doi: 10.1177/1010428319880080. Tumour Biol. 2019. PMID: 31603389
-
Allelopathic Effect of Calotropis procera, Hyoscyamus muticus and Pulicaria undulata Extracts on Seed Germination of Portulaca oleracea and Chenopodium murale.Pak J Biol Sci. 2020 Jan;23(10):1260-1266. doi: 10.3923/pjbs.2020.1260.1266. Pak J Biol Sci. 2020. PMID: 32981259
-
Structural perturbation of alpha-crystallin and its chaperone-like activity.Int J Biol Macromol. 1998 May-Jun;22(3-4):271-81. doi: 10.1016/s0141-8130(98)00025-7. Int J Biol Macromol. 1998. PMID: 9650082 Review.
-
Quantification of anti-aggregation activity of chaperones.Int J Biol Macromol. 2017 Jul;100:104-117. doi: 10.1016/j.ijbiomac.2016.07.066. Epub 2016 Jul 22. Int J Biol Macromol. 2017. PMID: 27456122 Review.
Cited by
-
A Novel Peptide Mapping Method Utilizing Cysteine as a Reducing Agent.Pharm Res. 2025 Jan;42(1):173-184. doi: 10.1007/s11095-024-03805-z. Epub 2025 Jan 23. Pharm Res. 2025. PMID: 39849215
References
-
- Boulos L. Flora of Egypt. Cairo, Egypt: Al-Hadara; 2002.
-
- Al-Rawi A. Flora of Kuwait. Oxford: Alden; 1987.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

