Immune responses to Vi capsular polysaccharide typhoid vaccine in children 2 to 16 years old in Karachi, Pakistan, and Kolkata, India
- PMID: 24599532
- PMCID: PMC4018880
- DOI: 10.1128/CVI.00791-13
Immune responses to Vi capsular polysaccharide typhoid vaccine in children 2 to 16 years old in Karachi, Pakistan, and Kolkata, India
Abstract
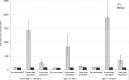
The geometric mean concentration (GMC) and the proportion maintaining a protective level (150 enzyme-linked immunosorbent assay (ELISA) units [ELU]/ml) 2 years following a single dose of 25 μg of injectable Vi capsular polysaccharide typhoid vaccine was measured against that of the control hepatitis A vaccine in children 2 to 16 years old in cluster randomized trials in Karachi and Kolkata. The GMC for the Vi group (1,428 ELU/ml) was statistically significantly different from the GMC of the control hepatitis A vaccine group (86 ELU/ml) after 6 weeks. A total of 117 children (95.1%) in the Vi group and 9 (7.5%) in the hepatitis A group showed a 4-fold rise in Vi IgG antibody concentrations at 6 weeks (P < 0.01). Protective antibody levels remained significantly different between the two groups at 2 years (38% in the Vi vaccine groups and 6% in the hepatitis A group [P < 0.01]). A very small proportion of younger children (2 to 5 years old) maintained protective Vi IgG antibody levels at 2 years, a result that was not statistically significantly different compared to that for the hepatitis A group (38.1% versus 10.5%). The GMCs of the Vi IgG antibody after 2 years were 133 ELU/ml for children 2 to <5 years old and 349 ELU/ml for children 5 to 16 years old. In conclusion, Vi capsular polysaccharide typhoid vaccine is immunogenic in children in settings of South Asia where typhoid is highly endemic. The antibody levels in children who received this vaccine remained higher than those in children who received the control vaccine but were significantly reduced at 2 years of follow-up.
Figures
References
-
- Breiman RF, Cosmas L, Njuguna H, Audi A, Olack B, Ochieng JB, Wamola N, Bigogo GM, Awiti G, Tabu CW. 2012. Population-based incidence of typhoid fever in an urban informal settlement and a rural area in Kenya: implications for typhoid vaccine use in Africa. PLoS One 7:e29119. 10.1371/journal.pone.0029119 - DOI - PMC - PubMed
-
- Punjabi NH, Agtini MD, Ochiai RL, Simanjuntak CH, Lesmana M, Subekti D, Oyofo BA, von Seidlein L, Deen J, Shin S, Acosta C, Wangsasaputra F, Pulungsih SP, Saroso S, Suyeti S, Sudarmono P, Syarurachman A, Suwandono A, Arjoso S, Beecham HJ, 3rd, Corwin AL, Clemens JD. 2013. Enteric fever burden in North Jakarta, Indonesia: a prospective, community-based study. J. Infect. Dev. Ctries. 7:781–787. 10.3855/jidc.2629 - DOI - PubMed
-
- Ochiai RL, Acosta CJ, Danovaro-Holliday MC, Baiqing D, Bhattacharya SK, Agtini MD, Bhutta ZA, Canh do G, Ali M, Shin S, Wain J, Page AL, Albert MJ, Farrar J, Abu-Elyazeed R, Pang T, Galindo CM, von Seidlein L, Clemens JD, Domi Typhoid Study Group 2008. A study of typhoid fever in five Asian countries: disease burden and implications for controls. Bull. World Health Organ. 86:260–268. 10.2471/BLT.06.039818 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources