Rapid generation of a mouse model for Middle East respiratory syndrome
- PMID: 24599590
- PMCID: PMC3977243
- DOI: 10.1073/pnas.1323279111
Rapid generation of a mouse model for Middle East respiratory syndrome
Abstract
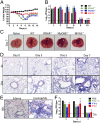
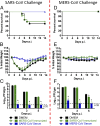
In this era of continued emergence of zoonotic virus infections, the rapid development of rodent models represents a critical barrier to public health preparedness, including the testing of antivirus therapy and vaccines. The Middle East respiratory syndrome coronavirus (MERS-CoV) was recently identified as the causative agent of a severe pneumonia. Given the ability of coronavirus to rapidly adapt to new hosts, a major public health concern is that MERS-CoV will further adapt to replication in humans, triggering a pandemic. No small-animal model for this infection is currently available, but studies suggest that virus entry factors can confer virus susceptibility. Here, we show that mice were sensitized to MERS-CoV infection by prior transduction with adenoviral vectors expressing the human host-cell receptor dipeptidyl peptidase 4. Mice developed a pneumonia characterized by extensive inflammatory-cell infiltration with virus clearance occurring 6-8 d after infection. Clinical disease and histopathological changes were more severe in the absence of type-I IFN signaling whereas the T-cell response was required for virus clearance. Using these mice, we demonstrated the efficacy of a therapeutic intervention (poly I:C) and a potential vaccine [Venezuelan equine encephalitis replicon particles expressing MERS-CoV spike protein]. We also found little protective cross-reactivity between MERS-CoV and the severe acute respiratory syndrome-CoV. Our results demonstrate that this system will be useful for MERS-CoV studies and for the rapid development of relevant animal models for emerging respiratory viral infections.
Keywords: SARS; emerging pathogen; interferon.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–1820. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

