Methods of preventing bacterial sepsis and wound complications after liver transplantation
- PMID: 24599680
- PMCID: PMC10882578
- DOI: 10.1002/14651858.CD006660.pub3
Methods of preventing bacterial sepsis and wound complications after liver transplantation
Abstract
Background: Bacterial sepsis and wound complications after liver transplantation increase mortality, morbidity, or hospital stay and are likely to increase overall transplant costs. All liver transplantation patients receive antibiotic prophylaxis. This is an update of our 2008 Cochrane systematic review on the same topic in which we identified seven randomised clinical trials.
Objectives: To assess the benefits and harms of different methods aimed at preventing bacterial sepsis and wound complications in people undergoing liver transplantation.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, and Science Citation Index Expanded to February 2013.
Selection criteria: We included only randomised clinical trials irrespective of language or publication status. We excluded quasi-randomised and other observational studies for assessment of benefits, but not for harms.
Data collection and analysis: Two review authors collected the data independently. We calculated the risk ratio (RR) or mean difference (MD) with 95% confidence intervals (CI) using fixed-effect and the random-effects models based on available-case analysis.
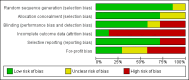
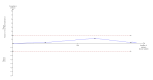
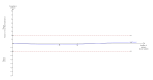
Main results: We identified only seven trials for inclusion, including 614 participants. Only one trial was of low risk of bias risk. Overall, the quality of evidence was very low. There were five comparisons in the seven trials: selective bowel decontamination versus inactive control; selective bowel decontamination versus prebiotics with probiotics; selective bowel decontamination versus prebiotics; prebiotics with probiotics versus prebiotics; and granulocyte-colony stimulating factor (G-CSF) versus control. Four trials compared selective bowel decontamination versus placebo or no treatment. In one trial, participants were randomised to selective bowel decontamination, active lactobacillus with fibres (probiotic with prebiotic), or to inactivated lactobacillus with fibres (prebiotic). In one trial, active lactobacillus with fibres (probiotic with prebiotic) was compared with inactive lactobacillus with fibres (prebiotic). In the remaining trial, different doses of G-CSF and placebo were compared. There was no trial comparing different antibiotic prophylactic regimens in people undergoing liver transplantation. Most trials included adults undergoing elective liver transplantation. There was no significant difference in proportion of people who died or required retransplantation between the intervention and control groups in any of the five comparison groups. MORTALITY There were no differences between 190 participants (three trials); 5/87 (adjusted proportion: 6.2%) in selective bowel decontamination group versus 7/103 (6.8%) in inactive control group; RR 0.91 (95% CI 0.31 to 2.72); 63 participants (one trial); 0/32 (0%) in selective bowel decontamination group versus 0/31 (0%) in prebiotics with probiotics group; RR - not estimable; 64 participants (one trial); 0/32 (0%) in selective bowel decontamination group versus 0/32 (0%) in prebiotics group; RR - not estimable; 129 participants (two trials); 0/64 (0%) in prebiotics with probiotics group versus 0/65 (0%) in prebiotics group; RR - not estimable; and 194 participants (one trial); 22/124 (17.7%) in G-CSF group versus 10/70 (14.3%) in placebo group; RR 1.24 (95% 0.62 to 2.47). RETRANSPLANTATION There were no differences between 132 participants (two trials); 4/58 (adjusted proportion: 6.9%) in selective bowel decontamination group versus 6/74 (8.1%) in inactive control group; RR 0.85 (95% CI 0.26 to 2.85); 63 participants (one trial); 1/32 (3.1%) in selective bowel decontamination group versus 0/31 (0%) in prebiotics with probiotics group; RR 2.91 (0.12 to 68.81); 64 participants (one trial); 1/32 (3.1%) in selective bowel decontamination group versus 0/32 (0%) in prebiotics group; RR 3.00 (95% CI 0.13 to 71.00); 129 participants (two trials); 0/64 (0%) in prebiotics with probiotics group versus 1/65 (1.5%) in prebiotics group; RR 0.33 (95% CI 0.01 to 7.9); and 194 participants (one trial); 10/124 (7.1%) in G-CSF group versus 5/70 (7.1%) in placebo group; RR 1.13 (95% CI 0.4 to 3.17).There was no significant difference in the graft rejections, intensive therapy unit stay, or hospital stay between the intervention and control groups in any of the comparisons. Overall, 193/611 participants (31.6%) developed infective complications. The proportion of people who developed infective complications and the number of infective complication episodes were significantly higher in the selective bowel decontamination group than in the prebiotics with probiotics group (1 study; 63 participants; 15/32 (46.9%) in selective bowel decontamination group versus 4/31 (12.9%) in prebiotics with probiotics group; RR 3.63; 95% CI 1.36 to 9.74 and 23/32 participants (0.72 infective complications per participant) in selective bowel decontamination group versus 4/31 participants (0.13 infective complications per participant) in prebiotics with probiotics group; rate ratio 5.58; 95% CI 1.94 to 16.09). There was no significant difference between the proportion of participants who developed infection and the number of infection episodes between the intervention group and control group in any of the other comparisons.No trials reported quality of life and overall serious adverse events.
Authors' conclusions: Currently, there is no clear evidence for any intervention offering significant benefits in the reduction of bacterial infections and wound complications in liver transplantation. Selective bowel decontamination may even increase the rate of infections compared with prebiotics with probiotics. The confidence intervals were wide and further randomised clinical trials of low risk of bias are necessary.
Conflict of interest statement
None known.
Figures











Update of
-
Methods of preventing bacterial sepsis and wound complications for liver transplantation.Cochrane Database Syst Rev. 2008 Oct 8;(4):CD006660. doi: 10.1002/14651858.CD006660.pub2. Cochrane Database Syst Rev. 2008. Update in: Cochrane Database Syst Rev. 2014 Mar 05;(3):CD006660. doi: 10.1002/14651858.CD006660.pub3. PMID: 18843724 Updated.
Comment in
-
What do Cochrane systematic reviews say about probiotics as preventive interventions?Sao Paulo Med J. 2017 Nov-Dec;135(6):578-586. doi: 10.1590/1516-3180.2017.0310241017. Sao Paulo Med J. 2017. PMID: 29267517 Free PMC article.
References
References to studies included in this review
Arnow 1996 {published data only}
-
- Arnow PM, Carandang GC, Zabner R, Irwin ME. Randomized controlled trial of selective bowel decontamination for prevention of infections following liver transplantation. Clinical Infectious Diseases 1996;22(6):997‐1003. - PubMed
Bion 1994 {published data only}
-
- Badger IL, Crosby HA, Kong KL, Baker JP, Hutchings P, Elliott TS, et al. Is selective decontamination of the digestive tract beneficial in liver transplant patients? Interim results of a prospective, randomized trial. Transplantation Proceedings 1991;23(1 Part 2):1460‐1. - PubMed
-
- Bion JF, Badger I, Crosby HA, Hutchings P, Kong KL, Baker J, et al. Selective decontamination of the digestive tract reduces gram‐negative pulmonary colonization but not systemic endotoxemia in patients undergoing elective liver transplantation. Critical Care Medicine 1994;22(1):40‐9. - PubMed
Hellinger 2002 {published and unpublished data}
-
- Hellinger WC, Yao JD, Alvarez S, Blair JE, Cawley JJ, Paya CV, et al. A randomized, prospective, double‐blinded evaluation of selective bowel decontamination in liver transplantation. Transplantation 2002;73(12):1904‐9. - PubMed
Rayes 2002 {published and unpublished data}
-
- Rayes N, Brammer M, Hansen S, Mueller AR, Serke S, Seehofer D, et al. Early enteral supply of lactobacilli and fiber versus SBD ‐ a prospective randomized trial in liver transplant recipients (abstract). Journal of Hepatology 2001;34(1):199.
-
- Rayes N, Seehofer D, Hansen S, Boucsein K, Muller AR, Serke S, et al. Early enteral supply of lactobacillus and fiber versus selective bowel decontamination: a controlled trial in liver transplant recipients. Transplantation 2002;74(1):123‐7. - PubMed
-
- Rayes N, Seehofer D, Muller AR, Hansen S, Bengmark S, Neuhaus P. Influence of probiotics and fibre on the incidence of bacterial infections following major abdominal surgery ‐ results of a prospective trial. Zeitschrift für Gastroenterologie 2002;40(10):869‐76. - PubMed
Rayes 2005 {published and unpublished data}
-
- Rayes N, Seehofer D, Theruvath T, Schiller RA, Langrehr JM, Jonas S, et al. Supply of pre‐ and probiotics reduces bacterial infection rates after liver transplantation ‐ a randomized, double‐blind trial. American Journal of Transplantation 2005;5(1):125‐30. - PubMed
Winston 1999 {published data only}
-
- Winston DJ, Foster PF, Somberg KA, Busuttil RW, Levy MF, Sheiner PA, et al. Randomized, placebo‐controlled, double‐blind, multicenter trial of efficacy and safety of granulocyte colony‐stimulating factor in liver transplant recipients. Transplantation 1999;68(9):1298‐304. - PubMed
Zwaveling 2002 {published data only}
-
- Maring JK, Zwaveling JH, Klompmaker IJ, Meer J, Slooff MJ. Selective bowel decontamination in elective liver transplantation: no improvement in endotoxaemia, initial graft function and post‐operative morbidity. Transplant International 2002;15(7):329‐34. - PubMed
-
- Zwaveling JH, Maring JK, Klompmaker IJ, Haagsma EB, Bottema JT, Laseur M, et al. Selective decontamination of the digestive tract to prevent postoperative infection: a randomized placebo‐controlled trial in liver transplant patients. Critical Care Medicine 2002;30(6):1204‐9. - PubMed
-
- Enckevort PJ, Zwaveling JH, Bottema JT, Maring JK, Klompmaker IJ, Slooff MJH, et al. Cost effectiveness of selective decontamination of the digestive tract in liver transplant patients. Pharmacoeconomics 2001;19(5):523‐30. - PubMed
References to studies excluded from this review
Biasi 2002 {published data only}
-
- Biasi F, Poli G, Salizzoni M, Cerutti E, Battista S, Mengozzi G, et al. Effect of perioperative infusion of antioxidants on neutrophil activation during liver transplantation in humans. Transplantation Proceedings 2002;34(3):755‐8. - PubMed
Cisneros 1993 {published data only}
-
- Cisneros AC, Sanchez IRJA, Montejo GJC, Arribas LP, Martinez DLGA. Infections in the post‐surgical period of liver transplantation. Comparison of two protocols with anti‐infective prophylaxis. Revista Clinica Espanola 1993;192(3):112‐5. - PubMed
Decruyenaere 1995 {published data only}
-
- Decruyenaere J, Colardyn F, Vogelaers D, Claeys G, Hesse U, Deyne C, et al. Combined use of fluconazole and selective digestive decontamination in the prevention of fungal infection after adult liver transplantation. Transplantation Proceedings 1995;27(6):3515‐6. - PubMed
Hasse 1995 {published data only}
-
- Hasse JM, Blue LS, Liepa GU, Goldstein RM, Jennings LW, Mor E, et al. Early enteral nutrition support in patients undergoing liver transplantation. Journal of Parenteral and Enteral Nutrition 1995;19(6):437‐43. - PubMed
Ott 2006 {published data only}
-
- Ott R, Bussenius‐Kammerer M, Reck T, Hering C, Muller V, Riese J, et al. Does the inactivation of leukocytes in blood transfusions during and following liver transplantation by gamma‐irradiation have an impact on rejection and infection rate?. Medical Science Monitor 2006;12(12):514‐20. - PubMed
Philpott‐Howard 2003 {published data only}
-
- Philpott‐Howard J, Burroughs A, Fisher N, Hastings M, Kibbler C, Mutimer D, et al. Piperacillin‐tazobactam versus ciprofloxacin plus amoxicillin in the treatment of infective episodes after liver transplantation. Journal of Antimicrobial Chemotherapy 2003;52(6):993‐1000. - PubMed
Smith 1993 {published data only}
-
- Smith SD, Jackson RJ, Hannakan CJ, Wadowsky RM, Tzakis AG, Rowe MI. Selective decontamination in pediatric liver transplants. A randomized prospective study. Transplantation 1993;55(6):1306‐9. - PubMed
van Saene 2003 {published data only}
-
- Saene HK, Silvestri L, Bams JL, Voort PH, Zandstra DF. Selective decontamination of the digestive tract: use in liver transplantation is evidence based. Critical Care Medicine 2003;31(5):1600‐1. - PubMed
Additional references
Agopian 2013
-
- Agopian VG, Petrowsky H, Kaldas FM, Zarrinpar A, Farmer DG, Yersiz H, et al. The evolution of liver transplantation during 3 decades: analysis of 5347 consecutive liver transplants at a single center. Annals of Surgery 2013;258(3):409‐21. - PubMed
Blair 2005
-
- Blair JE, Kusne S. Bacterial, mycobacterial, and protozoal infections after liver transplantation ‐ part I. Liver Transplantation 2005;11(12):1452‐9. - PubMed
Bombuy 2004
-
- Bombuy E, Fondevila C, Rodriguez‐Laiz G, Ferrer J, Amador A, Valentini M, et al. Ischemic preconditioning in adult living donor liver transplantation, a pilot study [EASL abstract]. Journal of Hepatology 2004;40(Suppl 1):39.
Bonnel 2011
-
- Bonnel AR, Bunchorntavakul C, Reddy KR. Immune dysfunction and infections in patients with cirrhosis. Clinical Gastroenterology and Hepatology 2011;9(9):727‐38. - PubMed
Bratzler 2013
-
- Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. American Journal of Health‐system Pharmacy 2013;70(3):195‐283. - PubMed
Brok 2008
-
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61:763‐9. - PubMed
Brok 2009
-
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. - PubMed
Cescon 2006
-
- Cescon M, Grazi GL, Grassi A, Ravaioli M, Vetrone G, Ercolani G, et al. Effect of ischemic preconditioning in whole liver transplantation from deceased donors. A pilot study. Liver Transplantation 2006;12(4):628‐35. - PubMed
Cholongitas 2006
-
- Cholongitas E, Marelli L, Shusang V, Senzolo M, Rolles K, Patch D, et al. A systematic review of the performance of the model for end‐stage liver disease (MELD) in the setting of liver transplantation. Liver Transplantation 2006;12(7):1049‐61. - PubMed
Corno 2006
-
- Corno V, Colledan M, Dezza MC, Guizzetti M, Lucianetti A, Maldini G, et al. Extended right split liver graft for primary transplantation in children and adults. Transplantation International 2006;19(6):492‐9. - PubMed
CTU 2011
-
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis, 2011. ctu.dk/tsa/ (accessed 4 March 2014).
De Groote 2005
-
- Groote MA, Frank DN, Dowell E, Glode MP, Pace NR. Lactobacillus rhamnosus GG bacteremia associated with probiotic use in a child with short gut syndrome. Pediatric Infectious Disease Journal 2005;24(3):278‐80. - PubMed
DeMets 1987
-
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
Diener 2010
-
- Diener MK, Voss S, Jensen K, Buchler MW, Seiler CM. Elective midline laparotomy closure: the INLINE systematic review and meta‐analysis. Annals of Surgery 2010;251(5):843‐56. - PubMed
Egger 1997
Garbino 2005
-
- Garbino J, Romand JA, Pittet D, Giostra E, Mentha G, Suter P. Infection and rejection in liver transplant patients: a 10‐year Swiss single‐centre experience. Swiss Medical Weekly 2005;135(39‐40):587‐93. - PubMed
Gluud 2013
-
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2013, Issue 5. Art. No.: LIVER.
Gurusamy 2009
-
- Gurusamy KS, Gluud C, Nikolova D, Davidson BR. Assessment of risk of bias in randomized clinical trials in surgery. British Journal of Surgery 2009;96(4):342‐9. [PUBMED: 19283747] - PubMed
Haddad 2006
Higgins 2002
-
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hodson 2005
ICH‐GCP 1997
-
- International Conference on Harmonisation Expert Working Group. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH harmonised tripartite guideline. Guideline for good clinical practice CFR & ICH guidelines. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997.
Kamath 2001
-
- Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end‐stage liver disease. Hepatology 2001;33(2):464‐70. - PubMed
Kato 2005
-
- Kato T, Yoshida H, Sadfar K, Martinez E, Nishida S, Moon J, et al. Steroid‐free induction and preemptive antiviral therapy for liver transplant recipients with hepatitis C: a preliminary report from a prospective randomized study. Transplant Proceedings 2005;37(2):1217‐9. - PubMed
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. - PubMed
Koneru 2005
-
- Koneru B, Fisher A, He Y, Klein KM, Skurnick J, Wilson DJ, et al. Ischemic preconditioning in deceased donor liver transplantation: a prospective randomized clinical trial of safety and efficacy. Liver Transplantation 2005;11(2):196‐202. - PubMed
Lim 2006
-
- Lim SG, Wai CT, Costa M, Sutedja DS, Lee YM, Lee KH, et al. Referral patterns and waiting times for liver transplantation in Singapore. Singapore Medical Journal 2006;47(7):599‐603. - PubMed
Lundh 2012
Macaskill 2001
-
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. - PubMed
Mehrabi 2006
-
- Mehrabi A, Fonouni H, Wente M, Sadeghi M, Eisenbach C, Encke J, et al. Wound complications following kidney and liver transplantation. Clinical Transplantation 2006;20(Suppl 17):97‐110. - PubMed
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. - PubMed
Newell 1992
-
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. - PubMed
NHS UK transplant
-
- NHS UK Transplant ‐ Annual Report 2003‐2004. www.organdonation.nhs.uk/statistics/transplant_activity_report/current_a... (accessed 4 March 2014).
OPTN/SRTR 2009
-
- Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR). OPTN/SRTR 2009 Annual Report. www.ustransplant.org/annual_reports/current/905_li.pdf (accessed 4 March 2014).
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [PUBMED: 9921604] - PubMed
Patel 1997
Playford 2004
Rautio 1999
-
- Rautio M, Jousimies‐Somer H, Kauma H, Pietarinen I, Saxelin M, Tynkkynen S, et al. Liver abscess due to a Lactobacillus rhamnosus strain indistinguishable from L. rhamnosus strain GG. Clinical Infectious Diseases 1999;28(5):1159‐60. - PubMed
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Royle 2003
-
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. - PubMed
Safdar 2004
-
- Safdar N, Said A, Lucey MR. The role of selective digestive decontamination for reducing infection in patients undergoing liver transplantation: a systematic review and meta‐analysis. Liver Transplantation 2004;10(7):817‐27. - PubMed
Savović 2012
-
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Health Technology Assessment 2012;16(35):1‐82. - PubMed
Savović 2012a
-
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. - PubMed
Shiffman 2006
-
- Shiffman ML, Saab S, Feng S, Abecassis MI, Tzakis AG, Goodrich NP, et al. Liver and intestine transplantation in the United States, 1995‐2004. American Journal of Transplantation 2006;6(5 Pt 2):1170‐87. - PubMed
SPIRIT 2013
SPIRIT 2013a
Strippoli 2006
Thorlund 2009
-
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses. International Journal of Epidemiology 2009;38(1):276‐86. - PubMed
Thorlund 2010
Thorlund 2011
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA), 2011. ctu.dk/tsa/files/tsa_manual.pdf (accessed 4 March 2014).
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. - PubMed
Wetterslev 2009
References to other published versions of this review
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

