Host microRNA regulation of human cytomegalovirus immediate early protein translation promotes viral latency
- PMID: 24599990
- PMCID: PMC4019081
- DOI: 10.1128/JVI.00481-14
Host microRNA regulation of human cytomegalovirus immediate early protein translation promotes viral latency
Abstract
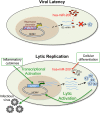
Reactivation of human cytomegalovirus (HCMV) is a significant cause of disease and death in immunocompromised patients, underscoring the need to understand how latency is controlled. Here we demonstrate that HCMV has evolved to utilize cellular microRNAs (miRNAs) in cells that promote latency to regulate expression of a viral protein critical for viral reactivation. Our data reveal that hsa-miR-200 miRNA family members target the UL122 (immediate early protein 2) 3' untranslated region, resulting in repression of this viral protein. Utilizing recombinant viruses that mutate the miRNA-binding site compared to the sequence of the wild-type virus results in lytic rather than latent infections in ex vivo infections of primary CD34+ cells. Cells permissive for lytic replication demonstrate low levels of these miRNAs. We propose that cellular miRNA regulation of HCMV is critical for maintenance of viral latency.
Importance: Human cytomegalovirus (HCMV) is a herpesvirus that infects a majority of the population. Once acquired, individuals harbor the virus for life, where the virus remains, for the most part, in a quiet or latent state. Under weakened immune conditions, the virus can reactivate, which can cause severe disease and often death. We have found that members of a family of small RNAs, termed microRNAs, encoded by human myeloid progenitor cells are capable of repressing a key viral protein, thus enabling the virus to ensure a quiet/latent state. As these progenitor cells mature further down the myeloid lineage toward cells that support active viral replication, the levels of these microRNAs decrease. Together, our data suggest that host cell microRNA regulation of HCMV is important for the quiet/latent state of this pathogen.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

