Combined adenovirus vector and hepatitis C virus envelope protein prime-boost regimen elicits T cell and neutralizing antibody immune responses
- PMID: 24599994
- PMCID: PMC4019094
- DOI: 10.1128/JVI.03574-13
Combined adenovirus vector and hepatitis C virus envelope protein prime-boost regimen elicits T cell and neutralizing antibody immune responses
Abstract
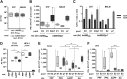
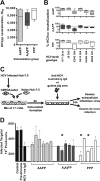
Despite the recent progress in the development of new antiviral agents, hepatitis C virus (HCV) infection remains a major global health problem, and there is a need for a preventive vaccine. We previously reported that adenoviral vectors expressing HCV nonstructural proteins elicit protective T cell responses in chimpanzees and were immunogenic in healthy volunteers. Furthermore, recombinant HCV E1E2 protein formulated with adjuvant MF59 induced protective antibody responses in chimpanzees and was immunogenic in humans. To develop an HCV vaccine capable of inducing both T cell and antibody responses, we constructed adenoviral vectors expressing full-length and truncated E1E2 envelope glycoproteins from HCV genotype 1b. Heterologous prime-boost immunization regimens with adenovirus and recombinant E1E2 glycoprotein (genotype 1a) plus MF59 were evaluated in mice and guinea pigs. Adenovirus prime and protein boost induced broad HCV-specific CD8+ and CD4+ T cell responses and functional Th1-type IgG responses. Immune sera neutralized luciferase reporter pseudoparticles expressing HCV envelope glycoproteins (HCVpp) and a diverse panel of recombinant cell culture-derived HCV (HCVcc) strains and limited cell-to-cell HCV transmission. This study demonstrated that combining adenovirus vector with protein antigen can induce strong antibody and T cell responses that surpass immune responses achieved by either vaccine alone.
Importance: HCV infection is a major health problem. Despite the availability of new directly acting antiviral agents for treating chronic infection, an affordable preventive vaccine provides the best long-term goal for controlling the global epidemic. This report describes a new anti-HCV vaccine targeting the envelope viral proteins based on adenovirus vector and protein in adjuvant. Rodents primed with the adenovirus vaccine and boosted with the adjuvanted protein developed cross-neutralizing antibodies and potent T cell responses that surpassed immune responses achieved with either vaccine component alone. If combined with the adenovirus vaccine targeting the HCV NS antigens now under clinical testing, this new vaccine might lead to a stronger and broader immune response and to a more effective vaccine to prevent HCV infection. Importantly, the described approach represents a valuable strategy for other infectious diseases in which both T and B cell responses are essential for protection.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

