Utilization of glyphosate as phosphate source: biochemistry and genetics of bacterial carbon-phosphorus lyase
- PMID: 24600043
- PMCID: PMC3957732
- DOI: 10.1128/MMBR.00040-13
Utilization of glyphosate as phosphate source: biochemistry and genetics of bacterial carbon-phosphorus lyase
Abstract
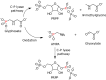
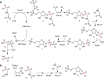
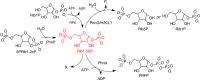
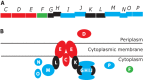
After several decades of use of glyphosate, the active ingredient in weed killers such as Roundup, in fields, forests, and gardens, the biochemical pathway of transformation of glyphosate phosphorus to a useful phosphorus source for microorganisms has been disclosed. Glyphosate is a member of a large group of chemicals, phosphonic acids or phosphonates, which are characterized by a carbon-phosphorus bond. This is in contrast to the general phosphorus compounds utilized and metabolized by microorganisms. Here phosphorus is found as phosphoric acid or phosphate ion, phosphoric acid esters, or phosphoric acid anhydrides. The latter compounds contain phosphorus that is bound only to oxygen. Hydrolytic, oxidative, and radical-based mechanisms for carbon-phosphorus bond cleavage have been described. This review deals with the radical-based mechanism employed by the carbon-phosphorus lyase of the carbon-phosphorus lyase pathway, which involves reactions for activation of phosphonate, carbon-phosphorus bond cleavage, and further chemical transformation before a useful phosphate ion is generated in a series of seven or eight enzyme-catalyzed reactions. The phn genes, encoding the enzymes for this pathway, are widespread among bacterial species. The processes are described with emphasis on glyphosate as a substrate. Additionally, the catabolism of glyphosate is intimately connected with that of aminomethylphosphonate, which is also treated in this review. Results of physiological and genetic analyses are combined with those of bioinformatics analyses.
Figures







References
-
- Shaner DL. 2009. Role of translocation as a mechanism of resistance to glyphosate. Weed Sci. 57:118–123. 10.1614/WS-08-050.1 - DOI
-
- Padgette SR, Kolacz KH, Delannay X, Re DB, LaVallee BJ, Tinius CN, Rhodes WK, Otero YI, Barry GF, Eicholtz DA, Peschke VM, Nida DL, Taylor NB, Kishore GM. 1995. Development, identification, and characterization of a glyphosate-tolerant soybean line. Crop Sci. 35:1451–1461. 10.2135/cropsci1995.0011183X003500050032x - DOI
-
- Comai L, Facciotti D, Hiatt WR, Thompson G, Rose RE, Stalker DM. 1985. Expression in plants of a mutant aroA gene from Salmonella typhimurium confers tolerance to glyphosate. Nature 317:441–444
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

