Cochlear neuropathy and the coding of supra-threshold sound
- PMID: 24600357
- PMCID: PMC3930880
- DOI: 10.3389/fnsys.2014.00026
Cochlear neuropathy and the coding of supra-threshold sound
Abstract
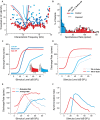
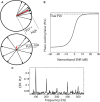
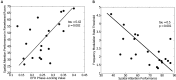
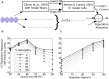
Many listeners with hearing thresholds within the clinically normal range nonetheless complain of difficulty hearing in everyday settings and understanding speech in noise. Converging evidence from human and animal studies points to one potential source of such difficulties: differences in the fidelity with which supra-threshold sound is encoded in the early portions of the auditory pathway. Measures of auditory subcortical steady-state responses (SSSRs) in humans and animals support the idea that the temporal precision of the early auditory representation can be poor even when hearing thresholds are normal. In humans with normal hearing thresholds (NHTs), paradigms that require listeners to make use of the detailed spectro-temporal structure of supra-threshold sound, such as selective attention and discrimination of frequency modulation (FM), reveal individual differences that correlate with subcortical temporal coding precision. Animal studies show that noise exposure and aging can cause a loss of a large percentage of auditory nerve fibers (ANFs) without any significant change in measured audiograms. Here, we argue that cochlear neuropathy may reduce encoding precision of supra-threshold sound, and that this manifests both behaviorally and in SSSRs in humans. Furthermore, recent studies suggest that noise-induced neuropathy may be selective for higher-threshold, lower-spontaneous-rate nerve fibers. Based on our hypothesis, we suggest some approaches that may yield particularly sensitive, objective measures of supra-threshold coding deficits that arise due to neuropathy. Finally, we comment on the potential clinical significance of these ideas and identify areas for future investigation.
Keywords: aging; auditory nerve; auditory steady-state response; frequency-following response; individual differences; noise-induced hearing loss; temporal coding; temporary threshold shift.
Figures

References
-
- Bharadwaj H. M., Masud M., Shinn-Cunningham B. G. (2013). Bottom-up and top-down contributions to individual differences in auditory spatial attention task performance. Presented at the Abstracts of the Midwinter Meeting of the ARO XXXVI: #887.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

