Morphological and bioenergetic demands underlying the mitophagy in post-mitotic neurons: the pink-parkin pathway
- PMID: 24600391
- PMCID: PMC3927396
- DOI: 10.3389/fnagi.2014.00018
Morphological and bioenergetic demands underlying the mitophagy in post-mitotic neurons: the pink-parkin pathway
Abstract
Evidence suggests a striking causal relationship between changes in quality control of neuronal mitochondria and numerous devastating human neurodegenerative diseases, including Parkinson's disease, Alzheimer's disease, Huntington's disease, and amyotrophic lateral sclerosis. Contrary to replicating mammalian cells with a metabolism essentially glycolytic, post-mitotic neurons are distinctive owing to (i) their exclusive energetic dependence from mitochondrial metabolism and (ii) their polarized shape, which entails compartmentalized and distinct energetic needs. Here, we review the recent findings on mitochondrial dynamics and mitophagy in differentiated neurons focusing on how the exceptional characteristics of neuronal populations in their morphology and bioenergetics needs make them quite different to other cells in controlling the intracellular turnover of these organelles.
Keywords: mitochondria dynamics; mitophagy; neurodegenerative diseases; primary neurons; quality control.
Figures
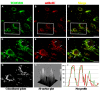

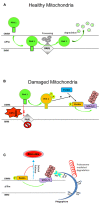
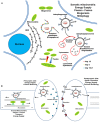
References
-
- Amadoro G., Corsetti V., Atlante A., Florenzano F., Capsoni S., Bussani R., et al. (2012). Interaction between NH(2)-tau fragment and Aβ in Alzheimer’s disease mitochondria contributes to the synaptic deterioration. Neurobiol. Aging 33 833.e1–833.e25 10.1016/j.neurobiolaging.2011.08.001 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

