Muscle hypertrophy is associated with increases in proteasome activity that is independent of MuRF1 and MAFbx expression
- PMID: 24600408
- PMCID: PMC3930915
- DOI: 10.3389/fphys.2014.00069
Muscle hypertrophy is associated with increases in proteasome activity that is independent of MuRF1 and MAFbx expression
Abstract
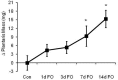
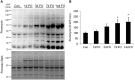
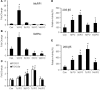
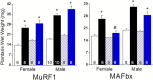
The regulation of skeletal muscle mass depends on the balance between protein synthesis and degradation. The role of protein degradation and in particular, the ubiquitin proteasome system, and increased expression of the E3 ubiquitin ligases, MuRF1 and MAFbx/atrogin-1, in the regulation of muscle size in response to growth stimuli is unclear. Thus, the aim of this study was to measure both proteasome activity and protein synthesis in mice over a 14-day period of chronic loading using the functional overload (FO) model. Further, the importance of MuRF1 and MAFbx expression in regulating muscle hypertrophy was examined by measuring muscle growth in response to FO in mice with a null deletion (KO) of either MuRF1 or MAFbx. In wild type (WT) mice, the increase in muscle mass correlated with significant increases (2-fold) in protein synthesis at 7 and 14 days. Interestingly, proteasome activity significantly increased in WT mice after one day, and continued to increase, peaking at 7 days following FO. The increase in proteasome activity was correlated with increases in the expression of the Forkhead transcription factors, FOXO1 and FOXO3a, which increased after both MuRF1 and MAFbx increased and returned to baseline. As in WT mice, hypertrophy in the MuRF1 and MAFbx KO mice was associated with significant increases in proteasome activity after 14 days of FO. The increase in plantaris mass was similar between the WT and MuRF1 KO mice following FO, however, muscle growth was significantly reduced in female MAFbx KO mice. Collectively, these results indicate that muscle hypertrophy is associated with increases in both protein synthesis and degradation. Further, MuRF1 or MAFbx expression is not required to increase proteasome activity following increased loading, however, MAFbx expression may be required for proper growth/remodeling of muscle in response to increase loading.
Keywords: forkhead transcription factors; functional overload; protein degradation; puromycin; ubiquitin proteasome system.
Figures


References
-
- Auclair D., Garrel D. R., Chaouki Zerouala A., Ferland L. H. (1997). Activation of the ubiquitin pathway in rat skeletal muscle by catabolic doses of glucocorticoids. Am. J. Physiol. 272, C1007–C1016 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

