Age- and sex-dependent effects of early life stress on hippocampal neurogenesis
- PMID: 24600436
- PMCID: PMC3929839
- DOI: 10.3389/fendo.2014.00013
Age- and sex-dependent effects of early life stress on hippocampal neurogenesis
Abstract
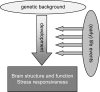
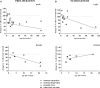
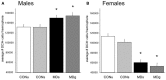
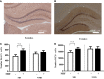
Early life stress is a well-documented risk factor for the development of psychopathology in genetically predisposed individuals. As it is hard to study how early life stress impacts human brain structure and function, various animal models have been developed to address this issue. The models discussed here reveal that perinatal stress in rodents exerts lasting effects on the stress system as well as on the structure and function of the brain. One of the structural parameters strongly affected by perinatal stress is adult hippocampal neurogenesis. Based on compiled literature data, we report that postnatal stress slightly enhances neurogenesis until the onset of puberty in male rats; when animals reach adulthood, neurogenesis is reduced as a consequence of perinatal stress. By contrast, female rats show a prominent reduction in neurogenesis prior to the onset of puberty, but this effect subsides when animals reach young adulthood. We further present preliminary data that transient treatment with a glucocorticoid receptor antagonist can normalize cell proliferation in maternally deprived female rats, while the compound had no effect in non-deprived rats. Taken together, the data show that neurogenesis is affected by early life stress in an age- and sex-dependent manner and that normalization may be possible during critical stages of brain development.
Keywords: adult neurogenesis; dentate gyrus; hippocampus; maternal deprivation; maternal separation; proliferation; rat; stress.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

