The challenges and importance of structural variation detection in livestock
- PMID: 24600474
- PMCID: PMC3927395
- DOI: 10.3389/fgene.2014.00037
The challenges and importance of structural variation detection in livestock
Abstract
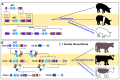
Recent studies in humans and other model organisms have demonstrated that structural variants (SVs) comprise a substantial proportion of variation among individuals of each species. Many of these variants have been linked to debilitating diseases in humans, thereby cementing the importance of refining methods for their detection. Despite progress in the field, reliable detection of SVs still remains a problem even for human subjects. Many of the underlying problems that make SVs difficult to detect in humans are amplified in livestock species, whose lower quality genome assemblies and incomplete gene annotation can often give rise to false positive SV discoveries. Regardless of the challenges, SV detection is just as important for livestock researchers as it is for human researchers, given that several productive traits and diseases have been linked to copy number variations (CNVs) in cattle, sheep, and pig. Already, there is evidence that many beneficial SVs have been artificially selected in livestock such as a duplication of the agouti signaling protein gene that causes white coat color in sheep. In this review, we will list current SV and CNV discoveries in livestock and discuss the problems that hinder routine discovery and tracking of these polymorphisms. We will also discuss the impacts of selective breeding on CNV and SV frequencies and mention how SV genotyping could be used in the future to improve genetic selection.
Keywords: CNVs; SV; antimicrobial peptides; coat color genetics; insertions and deletions (indels); livestock genomics; olfaction.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

