Hypoxia Affects the Structure of Breast Cancer Cell-Derived Matrix to Support Angiogenic Responses of Endothelial Cells
- PMID: 24600535
- PMCID: PMC3940068
- DOI: 10.4172/2157-2518.S13-005
Hypoxia Affects the Structure of Breast Cancer Cell-Derived Matrix to Support Angiogenic Responses of Endothelial Cells
Abstract
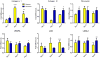
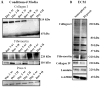
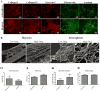
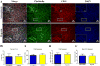
Hypoxia, a common feature of the tumor environment and participant in tumor progression, is known to alter gene and protein expression of several Extracellular Matrix (ECM) proteins, many of which have roles in angiogenesis. Previously, we reported that ECM deposited from co-cultures of Neonatal Fibroblasts (NuFF) with breast cancer cells, supported 3-dimensional vascular morphogenesis. Here, we sought to characterize the hypoxic ECM and to identify whether the deposited ECM induce angiogenic responses in Endothelial Cells (ECs). NuFF and MDA-MB-231 breast cancer cells were co-cultured, subjected to alternating cycles of 24 hours of 1% (hypoxia) and 21% (atmospheric) oxygen and de-cellularized for analyses of deposited ECM. We report differences in mRNA expression profiles of matrix proteins and crosslinking enzymes relevant to angiogenesis in hypoxia-exposed co-cultures. Interestingly, overt differences in the expression of ECM proteins were not detected in the de-cellularized ECM; however, up-regulation of the cell-binding fragment of fibronecin was observed in the conditioned media of hypoxic co-cultures. Ultrastructure analyses of the de-cellularized ECM revealed differences in fiber morphology with hypoxic fibers more compact and aligned, occupying a greater percent area and having larger diameter fibers than atmospheric ECM. Examining the effect of hypoxic ECM on angiogenic responses of ECs, morphological differences in Capillary-Like Structures (CLS) formed atop de-cellularized hypoxic and atmospheric ECM were not evident. Interestingly, we found that hypoxic ECM regulated the expression of angiogenic factors and matrix metalloproteinases in CLS. Overall, we report that in vitro, hypoxia does not alter the composition of the ECM deposited by co-cultures of NuFF/MDA-MB-231, but rather alters fiber morphology, and induces vascular expression of angiogenic growth factors and metalloproteinases. Taken together, these results have important implications for understanding how the hypoxic matrix may regulate angiogenesis in tumors.
Keywords: Angiogenesis; Extracellular matrix; Hypoxia.
Figures

Similar articles
-
Breast cancer cell-derived matrix supports vascular morphogenesis.Am J Physiol Cell Physiol. 2012 Apr 15;302(8):C1243-56. doi: 10.1152/ajpcell.00011.2012. Epub 2012 Jan 25. Am J Physiol Cell Physiol. 2012. PMID: 22277754 Free PMC article.
-
Fibronectin Deposition Participates in Extracellular Matrix Assembly and Vascular Morphogenesis.PLoS One. 2016 Jan 26;11(1):e0147600. doi: 10.1371/journal.pone.0147600. eCollection 2016. PLoS One. 2016. PMID: 26811931 Free PMC article.
-
Human lung fibroblast-derived matrix facilitates vascular morphogenesis in 3D environment and enhances skin wound healing.Acta Biomater. 2017 May;54:333-344. doi: 10.1016/j.actbio.2017.03.035. Epub 2017 Mar 27. Acta Biomater. 2017. PMID: 28351680
-
Breast tumor and stromal cell responses to TGF-β and hypoxia in matrix deposition.Matrix Biol. 2013 Mar 11;32(2):95-105. doi: 10.1016/j.matbio.2012.11.016. Epub 2012 Dec 20. Matrix Biol. 2013. PMID: 23262216 Free PMC article. Review.
-
Impaired angiogenesis in ageing: the central role of the extracellular matrix.J Transl Med. 2023 Jul 11;21(1):457. doi: 10.1186/s12967-023-04315-z. J Transl Med. 2023. PMID: 37434156 Free PMC article. Review.
Cited by
-
Progress towards understanding heterotypic interactions in multi-culture models of breast cancer.Integr Biol (Camb). 2016 Jun 13;8(6):684-92. doi: 10.1039/c6ib00001k. Epub 2016 Apr 21. Integr Biol (Camb). 2016. PMID: 27097801 Free PMC article. Review.
-
Transitions from mono- to co- to tri-culture uniquely affect gene expression in breast cancer, stromal, and immune compartments.Biomed Microdevices. 2016 Aug;18(4):70. doi: 10.1007/s10544-016-0083-x. Biomed Microdevices. 2016. PMID: 27432323 Free PMC article.
-
Hypoxic tumor microenvironment: Implications for cancer therapy.Exp Biol Med (Maywood). 2020 Jul;245(13):1073-1086. doi: 10.1177/1535370220934038. Epub 2020 Jun 27. Exp Biol Med (Maywood). 2020. PMID: 32594767 Free PMC article. Review.
-
The mechanistic immunosuppressive role of the tumour vasculature and potential nanoparticle-mediated therapeutic strategies.Front Immunol. 2022 Aug 15;13:976677. doi: 10.3389/fimmu.2022.976677. eCollection 2022. Front Immunol. 2022. PMID: 36045675 Free PMC article. Review.
-
Tumor hypoxia: From basic knowledge to therapeutic implications.Semin Cancer Biol. 2023 Jan;88:172-186. doi: 10.1016/j.semcancer.2022.12.011. Epub 2023 Jan 2. Semin Cancer Biol. 2023. PMID: 36603793 Free PMC article. Review.
References
-
- Liao D, Johnson RS. Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007;26:281–290. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
