Induced and pre-existing anti-polyethylene glycol antibody in a trial of every 3-week dosing of pegloticase for refractory gout, including in organ transplant recipients
- PMID: 24602182
- PMCID: PMC4060462
- DOI: 10.1186/ar4500
Induced and pre-existing anti-polyethylene glycol antibody in a trial of every 3-week dosing of pegloticase for refractory gout, including in organ transplant recipients
Abstract
Introduction: Pegloticase, a PEGylated recombinant porcine uricase, is approved for treating refractory gout at a dose of 8 mg intravenous (IV) every 2 weeks. However, during phase 1 testing, pharmacokinetics supported less frequent dosing. Also, single doses of pegloticase unexpectedly induced antibodies (Ab) that bound to polyethylene glycol (PEG). We have conducted a phase 2 trial to evaluate every 3-week dosing, and to further define the Ab response to pegloticase. Organ transplant recipients were included, as they are prone to severe gout that is difficult to manage, and because treatment to prevent graft rejection might influence the immune response to pegloticase.
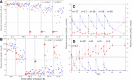
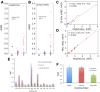
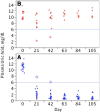
Methods: Plasma uricase activity (pUox), urate concentration (pUA), and clinical response were monitored during up to 5 infusions in 30 patients, including 7 organ transplant recipients. Depending on whether pUA <6 mg/dL was achieved and maintained, patients were classified as non (NR), persistent (PR), or transient (TR) responders. Ab to pegloticase and 10 kDa mPEG were monitored by enzyme linked immunosorbent assay and specificity was further defined.
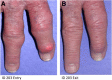
Results: We observed 17 PR, 12 TR, and 1 NR; 21 patients (16 PR, 5 TR) received all 5 infusions. Over the 15-week trial, pUA in PR averaged 1.0 ± 0.4 mg/dL; T½ for pUox was approximately 13 days, and area under the curve after dose 5 was approximately 30% higher than after dose 1. PR showed clinical benefit and in some, tophi resolved. In 11 of 12 TR, pUox fell rapidly and hyperuricemia recurred before dose 2. In all TR and NR, loss of response to pegloticase was accompanied by Ab to PEG, which was pre-existing in half of those who had no prior exposure to pegloticase. No PR, and 1 one out of 7 organ transplant recipients, had a sustained Ab response to pegloticase.
Conclusions: Every 3-week dosing is effective and may enhance the utility of pegloticase for treating refractory gout. Ab to PEG, which were pre-existing or induced by treatment, caused rapid loss of efficacy and increased the risk of infusion reactions. Organ transplant recipients can benefit from pegloticase, and may be less prone than non-recipients to developing anti-PEG Ab. Investigation of immunosuppressive strategies to minimize anti-PEG Ab is warranted.
Trial registration: ClincalTrials.gov identifier: NCT00111657.
Figures

Comment in
-
PEG-ing down (and preventing?) the cause of pegloticase failure.Arthritis Res Ther. 2014 May 30;16(3):112. doi: 10.1186/ar4572. Arthritis Res Ther. 2014. PMID: 25142440 Free PMC article.
References
-
- Becker MA, Schumacher HR, Benjamin KL, Gorevic P, Greenwald M, Fessel J, Edwards L, Kawata AK, Frank L, Waltrip R, Huang B, Sundy JS. Gout Natural History Study Group. Quality of life and disability in patients with treatment-failure gout. J Rheumatol. 2009;36:1041–1048. doi: 10.3899/jrheum.071229. - DOI - PubMed
-
- Sundy JS, Baraf HS, Yood RA, Edwards NL, Gutierrez-Urena SR, Treadwell EL, Vazquez-Mellado J, White WB, Lipsky PE, Horowitz Z, Huang W, Maroli AN, Waltrip RW 2nd, Hamburger SA, Becker MA. Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment: two randomized controlled trials. JAMA. 2011;306:711–720. - PubMed
-
- Krystexxa Prescribing Information. NDC#54396-801-01. http://krystexxa.com/pdfs/KRYSTEXXA_Prescribing_Information.pdf.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

