Skeletal and soft tissue response to automated, continuous, curvilinear distraction osteogenesis
- PMID: 24602810
- PMCID: PMC4101076
- DOI: 10.1016/j.joms.2014.01.004
Skeletal and soft tissue response to automated, continuous, curvilinear distraction osteogenesis
Abstract
Purpose: To document the bone formation and soft tissue changes in response to automated, continuous, curvilinear distraction osteogenesis (DO) at rates greater than 1 mm/day in a minipig model.
Materials and methods: Two groups of Yucatan minipigs underwent automated, continuous, curvilinear DO of the right mandible: group A, 1.5 mm/day (n = 5); and group B, 3.0 mm/day (n = 5). Each minipig underwent 12 mm of distraction followed by 24 days of fixation. The distracted and contralateral mandibles were harvested at the end of fixation. The percentage of surface area (PSA) of the regenerate occupied by bone, fibrous tissue, cartilage, and hematoma was determined using computerized histomorphometric analysis. The control groups consisted of DO wounds distracted discontinuously at 1 mm/day and the nonoperated contralateral mandible. The ipsilateral and contralateral digastric muscles were harvested and stained for proliferating cell nuclear antigen (PCNA), myogenic differentiation-1 (MyoD), and paired Box 7 protein (PAX7).
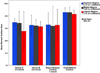
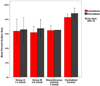
Results: All 10 minipigs completed the distraction and fixation period. The PSA occupied by bone was similar for groups A (PSA 64.36% ± 5.87%) and B (PSA 63.83% ± 3.37%) and the control group (1 mm/day; PSA 64.89% ± 0.56%) but was less than that on the nonoperated side (PSA 84.67% ± 0.86%). The PSA occupied by cartilage and hematoma in all groups was minimal (<1.1%). The digastric muscles had no abnormal tissue or inflammation, and PAX7, MyoD, and PCNA expression had returned to the baseline levels.
Conclusions: The results of the present study indicate that bone formation in response to automated, continuous, and curvilinear DO at a rate of 1.5 and 3.0 mm/day is nearly identical to that with discontinuous DO at 1 mm/day. In addition, no deleterious effects were found on the digastric muscles.
Copyright © 2014 American Association of Oral and Maxillofacial Surgeons. Published by Elsevier Inc. All rights reserved.
Figures






















References
-
- McCarthy JG, Schreiber J, Karp N, Thorne CH, Grayson BH. Lengthening the human mandible by gradual distraction. Plast Reconstr Surg. 1992;89:1. - PubMed
-
- Swennen G, Schliephake H, Dempf R, Schierle H, Malevez C. Craniofacial distraction osteogenesis: a review of the literature: Part 1: clinical studies. Int J Oral Maxillofac Surg. 2001;30:89. - PubMed
-
- Goldwaser BR, Magill J, Papadaki ME, Byl M, Kromann R, Yates B, Morency J, Kaban LB, Troulis MJ. Continuous mandibular distraction osteogenesis: novel device and preliminary results in minipigs. J Oral Maxillofac Surg. 2013;71:e168. - PubMed
-
- Ilizarov GA. The principles of the Ilizarov method. Bull Hosp Jt Dis Orthop Inst. 1988;48:1. - PubMed
-
- Ilizarov GA. The tension-stress effect on the genesis and growth of tissues: Part II. The influence of the rate and frequency of distraction. Clin Orthop Relat Res. 1989:263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

