Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial
- PMID: 24602844
- PMCID: PMC4144040
- DOI: 10.1016/S1473-3099(13)70692-3
Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial
Erratum in
- Lancet Infect Dis. 2014 Apr;14(4):269
Abstract
Background: Use of antiretroviral treatment for HIV-1 infection has decreased AIDS-related morbidity and mortality and prevents sexual transmission of HIV-1. However, the best time to initiate antiretroviral treatment to reduce progression of HIV-1 infection or non-AIDS clinical events is unknown. We reported previously that early antiretroviral treatment reduced HIV-1 transmission by 96%. We aimed to compare the effects of early and delayed initiation of antiretroviral treatment on clinical outcomes.
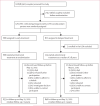
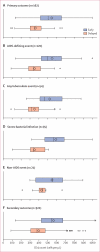
Methods: The HPTN 052 trial is a randomised controlled trial done at 13 sites in nine countries. We enrolled HIV-1-serodiscordant couples to the study and randomly allocated them to either early or delayed antiretroviral treatment by use of permuted block randomisation, stratified by site. Random assignment was unblinded. The HIV-1-infected member of every couple initiated antiretroviral treatment either on entry into the study (early treatment group) or after a decline in CD4 count or with onset of an AIDS-related illness (delayed treatment group). Primary events were AIDS clinical events (WHO stage 4 HIV-1 disease, tuberculosis, and severe bacterial infections) and the following serious medical conditions unrelated to AIDS: serious cardiovascular or vascular disease, serious liver disease, end-stage renal disease, new-onset diabetes mellitus, and non-AIDS malignant disease. Analysis was by intention-to-treat. This trial is registered with ClinicalTrials.gov, number NCT00074581.
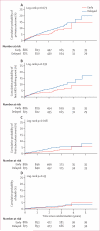
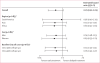
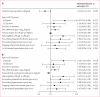
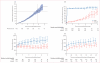
Findings: 1763 people with HIV-1 infection and a serodiscordant partner were enrolled in the study; 886 were assigned early antiretroviral treatment and 877 to the delayed treatment group (two individuals were excluded from this group after randomisation). Median CD4 counts at randomisation were 442 (IQR 373-522) cells per μL in patients assigned to the early treatment group and 428 (357-522) cells per μL in those allocated delayed antiretroviral treatment. In the delayed group, antiretroviral treatment was initiated at a median CD4 count of 230 (IQR 197-249) cells per μL. Primary clinical events were reported in 57 individuals assigned to early treatment initiation versus 77 people allocated to delayed antiretroviral treatment (hazard ratio 0·73, 95% CI 0·52-1·03; p=0·074). New-onset AIDS events were recorded in 40 participants assigned to early antiretroviral treatment versus 61 allocated delayed initiation (0·64, 0·43-0·96; p=0·031), tuberculosis developed in 17 versus 34 patients, respectively (0·49, 0·28-0·89, p=0·018), and primary non-AIDS events were rare (12 in the early group vs nine with delayed treatment). In total, 498 primary and secondary outcomes occurred in the early treatment group (incidence 24·9 per 100 person-years, 95% CI 22·5-27·5) versus 585 in the delayed treatment group (29·2 per 100 person-years, 26·5-32·1; p=0·025). 26 people died, 11 who were allocated to early antiretroviral treatment and 15 who were assigned to the delayed treatment group.
Interpretation: Early initiation of antiretroviral treatment delayed the time to AIDS events and decreased the incidence of primary and secondary outcomes. The clinical benefits recorded, combined with the striking reduction in HIV-1 transmission risk previously reported, provides strong support for earlier initiation of antiretroviral treatment.
Funding: US National Institute of Allergy and Infectious Diseases.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
Comment in
-
End of the debate about antiretroviral treatment initiation.Lancet Infect Dis. 2014 Apr;14(4):258-9. doi: 10.1016/S1473-3099(13)70329-3. Epub 2014 Mar 4. Lancet Infect Dis. 2014. PMID: 24602843 Free PMC article. No abstract available.
References
-
- Braitstein P, Brinkhof MW, Dabis F, et al. for the Antiretroviral Therapy in Lower Income Countries (ART-LINC) Collaboration, and ART Cohort Collaboration (ART-CC) groups Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–24. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UM1 AI069424/AI/NIAID NIH HHS/United States
- UM1 AI069456/AI/NIAID NIH HHS/United States
- UM1-AI068619/AI/NIAID NIH HHS/United States
- U01-AI068619/AI/NIAID NIH HHS/United States
- R37 DK049381/DK/NIDDK NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- UM1 AI069518/AI/NIAID NIH HHS/United States
- UM1 AI069453/AI/NIAID NIH HHS/United States
- UM1 AI069496/AI/NIAID NIH HHS/United States
- UM1 AI106701/AI/NIAID NIH HHS/United States
- UM1 AI068613/AI/NIAID NIH HHS/United States
- U01-AI068636/AI/NIAID NIH HHS/United States
- U01-AI068617/AI/NIAID NIH HHS/United States
- UM1 AI069423/AI/NIAID NIH HHS/United States
- U01-AI068613/AI/NIAID NIH HHS/United States
- U01 AI068619/AI/NIAID NIH HHS/United States
- U01 AI069463/AI/NIAID NIH HHS/United States
- UM1 AI069432/AI/NIAID NIH HHS/United States
- U19 AI053217/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- UM1 AI069463/AI/NIAID NIH HHS/United States
- U01 AI068617/AI/NIAID NIH HHS/United States
- U01 AI068613/AI/NIAID NIH HHS/United States
- UM1 AI068619/AI/NIAID NIH HHS/United States
- UM1 AI069436/AI/NIAID NIH HHS/United States
- UM1-AI068613/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
- UM1-AI068617/AI/NIAID NIH HHS/United States
- UM1 AI068617/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

