Sertoli cells--immunological sentinels of spermatogenesis
- PMID: 24603046
- PMCID: PMC4043859
- DOI: 10.1016/j.semcdb.2014.02.011
Sertoli cells--immunological sentinels of spermatogenesis
Abstract
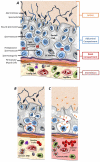
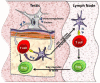
Testicular germ cells, which appear after the establishment of central tolerance, express novel cell surface and intracellular proteins that can be recognized as 'foreign antigens' by the host's immune system. However, normally these germ cells do not evoke an auto-reactive immune response. The focus of this manuscript is to review the evidence that the blood-testis-barrier (BTB)/Sertoli cell (SC) barrier along with the SCs ability to modulate the immune response is vital for protecting auto-antigenic germ cells. In normal testis, the BTB/SC barrier protects the majority of the auto-antigenic germ cells by limiting access by the immune system and sequestering these 'new antigens'. SCs also modulate testis immune cells (induce regulatory immune cells) by expressing several immunoregulatory factors, thereby creating a local tolerogenic environment optimal for survival of nonsequesetred auto-antigenic germ cells. Collectively, the fortress created by the BTB/SC barrier along with modulation of the immune response is pivotal for completion of spermatogenesis and species survival.
Keywords: Blood–testis-barrier; Immune privilege; Sertoli cells; Spermatogenesis; Testis.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures

References
-
- Dym M. The fine structure of the monkey (Macaca) Sertoli cell and its role in maintaining the blood-testis barrier. The Anatomical record. 1973;175:639–56. - PubMed
-
- Dym M, Fawcett DW. The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biology of reproduction. 1970;3:308–26. - PubMed
-
- Hess RA, Renato de Franca L. Spermatogenesis and cycle of the seminiferous epithelium. Advances in experimental medicine and biology. 2008;636:1–15. - PubMed
-
- O’Rand MG, Romrell LJ. Appearance of cell surface auto- and isoantigens during spermatogenesis in the rabbit. Developmental biology. 1977;55:347–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

