The first steps of adaptation of Escherichia coli to the gut are dominated by soft sweeps
- PMID: 24603313
- PMCID: PMC3945185
- DOI: 10.1371/journal.pgen.1004182
The first steps of adaptation of Escherichia coli to the gut are dominated by soft sweeps
Abstract
The accumulation of adaptive mutations is essential for survival in novel environments. However, in clonal populations with a high mutational supply, the power of natural selection is expected to be limited. This is due to clonal interference--the competition of clones carrying different beneficial mutations--which leads to the loss of many small effect mutations and fixation of large effect ones. If interference is abundant, then mechanisms for horizontal transfer of genes, which allow the immediate combination of beneficial alleles in a single background, are expected to evolve. However, the relevance of interference in natural complex environments, such as the gut, is poorly known. To address this issue, we have developed an experimental system which allows to uncover the nature of the adaptive process as Escherichia coli adapts to the mouse gut. This system shows the invasion of beneficial mutations in the bacterial populations and demonstrates the pervasiveness of clonal interference. The observed dynamics of change in frequency of beneficial mutations are consistent with soft sweeps, where different adaptive mutations with similar phenotypes, arise repeatedly on different haplotypes without reaching fixation. Despite the complexity of this ecosystem, the genetic basis of the adaptive mutations revealed a striking parallelism in independently evolving populations. This was mainly characterized by the insertion of transposable elements in both coding and regulatory regions of a few genes. Interestingly, in most populations we observed a complete phenotypic sweep without loss of genetic variation. The intense clonal interference during adaptation to the gut environment, here demonstrated, may be important for our understanding of the levels of strain diversity of E. coli inhabiting the human gut microbiota and of its recombination rate.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


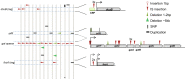
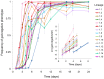

References
-
- Hegreness M, Shoresh N, Hartl D, Kishony R (2006) An equivalence principle for the incorporation of favorable mutations in asexual populations. Science 311: 1615–1617 doi:311/5767/1615 - PubMed
-
- Maharjan R, Seeto S, Notley-McRobb L, Ferenci T (2006) Clonal adaptive radiation in a constant environment. Science 313: 514–517 doi:10.1126/science.1129865 - DOI - PubMed
-
- Perfeito L, Fernandes L, Mota C, Gordo I (2007) Adaptive mutations in bacteria: high rate and small effects. Science 317: 813–815 doi:317/5839/813 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

