Extracellular ATP drives systemic inflammation, tissue damage and mortality
- PMID: 24603330
- PMCID: PMC3973196
- DOI: 10.1038/cddis.2014.70
Extracellular ATP drives systemic inflammation, tissue damage and mortality
Abstract
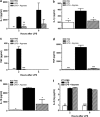
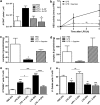
Systemic inflammatory response syndromes (SIRS) may be caused by both infectious and sterile insults, such as trauma, ischemia-reperfusion or burns. They are characterized by early excessive inflammatory cytokine production and the endogenous release of several toxic and damaging molecules. These are necessary to fight and resolve the cause of SIRS, but often end up progressively damaging cells and tissues, leading to life-threatening multiple organ dysfunction syndrome (MODS). As inflammasome-dependent cytokines such as interleukin-1β are critically involved in the development of MODS and death in SIRS, and ATP is an essential activator of inflammasomes in vitro, we decided to analyze the ability of ATP removal to prevent excessive tissue damage and mortality in a murine LPS-induced inflammation model. Our results indeed indicate an important pro-inflammatory role for extracellular ATP. However, the effect of ATP is not restricted to inflammasome activation at all. Removing extracellular ATP with systemic apyrase treatment not only prevented IL-1β accumulation but also the production of inflammasome-independent cytokines such as TNF and IL-10. In addition, ATP removal also prevented systemic evidence of cellular disintegration, mitochondrial damage, apoptosis, intestinal barrier disruption and even mortality. Although blocking ATP receptors with the broad-spectrum P2 purinergic receptor antagonist suramin imitated certain beneficial effects of apyrase treatment, it could not prevent morbidity or mortality at all. We conclude that removal of systemic extracellular ATP could be a valuable strategy to dampen systemic inflammatory damage and toxicity in SIRS.
Figures


References
-
- Angus DC. The search for effective therapy for sepsis: back to the drawing board. JAMA. 2011;306:2614–2615. - PubMed
-
- Harrois A, Huet O, Duranteau J. Alterations of mitochondrial function in sepsis and critical illness. Curr Opin Anaesthesiol. 2009;22:143–149. - PubMed
-
- Hernandez G, Bruhn A, Ince C. Microcirculation in sepsis: new perspectives. Curr Vasc Pharmacol. 2013;11:161–169. - PubMed
-
- Buchman TG, Cobb JP, Lapedes AS, Kepler TB. Complex systems analysis: a tool for shock research. Shock. 2001;16:248–251. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

