Systematic review of the use of dried blood spots for monitoring HIV viral load and for early infant diagnosis
- PMID: 24603442
- PMCID: PMC3945725
- DOI: 10.1371/journal.pone.0086461
Systematic review of the use of dried blood spots for monitoring HIV viral load and for early infant diagnosis
Abstract
Background: Dried blood spots (DBS) have been used as alternative specimens to plasma to increase access to HIV viral load (VL) monitoring and early infant diagnosis (EID) in remote settings. We systematically reviewed evidence on the performance of DBS compared to plasma for VL monitoring and EID.
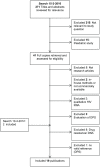
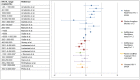
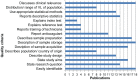
Methods and findings: Thirteen peer reviewed HIV VL publications and five HIV EID papers were included. Depending on the technology and the viral load distribution in the study population, the percentage of DBS samples that are within 0.5 log of VL in plasma ranged from 52-100%. Because the input sample volume is much smaller in a blood spot, there is a risk of false negatives with DBS. Sensitivity of DBS VL was found to be 78-100% compared to plasma at VL below 1000 copies/ml, but this increased to 100% at a threshold of 5000 copies/ml. Unlike a plasma VL test which measures only cell free HIV RNA, a DBS VL also measures proviral DNA as well as cell-associated RNA, potentially leading to false positive results when using DBS. The systematic review showed that specificity was close to 100% at DBS VL above 5000 copies/ml, and this threshold would be the most reliable for predicting true virologic failure using DBS. For early infant diagnosis, DBS has a sensitivity of 100% compared to fresh whole blood or plasma in all studies.
Conclusions: Although limited data are available for EID, DBS offer a highly sensitive and specific sampling strategy to make viral load monitoring and early infant diagnosis more accessible in remote settings. A standardized approach for sampling, storing, and processing DBS samples would be essential to allow successful implementation.
Trial registration: PROSPERO Registration #: CRD42013003621.
Conflict of interest statement
Figures

References
-
- UNAIDS (2012) Together we will end AIDS, Joing United Nations Progremme on HIV/AIDS: Geneva.
-
- World Health Organization (2011) Global Report 2011: Global HIV/AIDS response, WHO UNAIDS, Geneva.
-
- Murtagh M (2012)HIV/AIDS Diagnostics Technology Landscape: Semi-Annual Update. In: UNITAID, editor. Geneva: UNITAID.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

