Cost-effectiveness of Telaprevir combination therapy for chronic hepatitis C
- PMID: 24603445
- PMCID: PMC3946047
- DOI: 10.1371/journal.pone.0090295
Cost-effectiveness of Telaprevir combination therapy for chronic hepatitis C
Abstract
Objective: To explore the expected long-term health and economic outcomes of telaprevir (TVR) plus peginterferon alfa-2a and ribavirin (PR), a regimen that demonstrated substantially increased sustained virologic response (SVR) compared with PR alone in adults with chronic genotype 1 hepatitis C virus (HCV) and compensated liver disease in the Phase III studies ADVANCE (treatment-naïve patients) and REALIZE (relapsers, partial responders, and null responders to previous PR treatment).
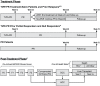
Study design: A decision-analytic model was developed to assess the cost-effectiveness of TVR+PR vs. PR in the United States (US).
Methods: Patients first moved through the 72-week decision-tree treatment phase of the model and then entered the cyclic Markov post-treatment phase. Clinical data (patient characteristics, SVR rates, and adverse event rates and durations) were obtained from ADVANCE and REALIZE. Health-state transition probabilities, drug and other costs (in 2012/2013 US dollars), and utility values were obtained from the trials, published studies, and publicly available sources. Outcomes were discounted at 3% per year.
Results: Regardless of treatment history, patients receiving TVR+PR were projected to experience fewer liver-disease complications, more life-years, and more quality-adjusted life-years (QALYs) than patients receiving PR. In prior relapsers, TVR+PR was dominant, with lower total medical costs and more QALYs. For the other patient subgroups, incremental costs per QALY gained were between $16,778 (treatment-naïve patients) and $34,279 (prior null responders). Extensive sensitivity analyses confirmed robust model results.
Conclusions: At standard willingness-to-pay thresholds, TVR+PR represents a cost-effective treatment option compared with PR alone for patients with chronic genotype 1 HCV and compensated liver disease in the US. Future analyses are needed to compare TVR+PR with all existing HCV treatment options.
Conflict of interest statement
Figures
References
-
- World Health Organization (WHO) (2011) Hepatitis C. Available: http://www.who.int/mediacentre/factsheets/fs164/en/. Accessed 10 February 2012.
-
- Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, et al. (2006) The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Int Med 144: 705–714. - PubMed
-
- Centers for Disease Control and Prevention (CDC) (2011) Hepatitis C information for health professionals: statistics and surveillance. Available: http://www.cdc.gov/hepatitis/HCV/StatisticsHCV.htm. Accessed 13 February 2012.
-
- Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW (2010) Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology 138: : 513–521, 521, e1–e6. - PubMed
-
- Thein HH, Yi Q, Dore GJ, Krahn MD (2008) Estimate of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology 48: 418–431. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

