Attitudes towards non-invasive prenatal testing for aneuploidy among US adults of reproductive age
- PMID: 24603453
- PMCID: PMC4399855
- DOI: 10.1038/jp.2014.30
Attitudes towards non-invasive prenatal testing for aneuploidy among US adults of reproductive age
Abstract
Objective: To determine how adults in the United States view non-invasive prenatal testing using cell-free fetal DNA (cffDNA testing) in order to help estimate uptake.
Study design: A national sample of 1861 US-based adults was surveyed using a validated online survey instrument. The survey was administered by a commercial survey research company. Respondents were randomized to receive a survey about prenatal testing for trisomy 13 and 18 or trisomy 21. Participants were asked to select among testing modalities, including cffDNA testing, and rank the features of testing that they considered most important to decision making.
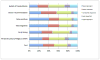
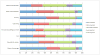
Result: There was substantive interest in the use of cffDNA testing rather than traditional screening mechanisms, with a minority of respondents reporting that they would support the use of both methods in combination. The lower rates of false-negative and false-positive test results and the ability to use the test earlier in the pregnancy were the most highly rated benefits of cffDNA testing. Participants expressed strong support for diagnostic confirmation via invasive testing after a positive result from either screening or cffDNA testing. However, almost one-third of participants reported that they would not endorse the use of either invasive or non-invasive prenatal testing.
Conclusion: There appears to be support for uptake of non-invasive prenatal tests. Clinical guidelines should therefore go forward in providing guidance on how to integrate non-invasive methods into the current standard of care. However, our findings indicate that even when accuracy, which is rated by patients as the most important aspect of prenatal testing, is significantly improved over existing screening methods and testing is offered non-invasively, the number of individuals who reported that they would decline any testing remained the same. Attention should therefore be directed at ensuring that the right of informed refusal of prenatal testing is not impacted by new, non-invasive methods.
Conflict of interest statement
Conflict of Interest: The authors declare no conflict of interest.
Figures


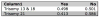
References
-
- Heger M. As rivals vie for share of noninvasive trisomy testing market, physicians see value for screening. GenomeWeb. 2012 Jul 3;
-
- Sayres LC, Cho MK. Cell-Free fetal nucleic acid testing: A review of the technology and its applications. Obstetrical & Gynecological Survey. 2011;66(7):431. - PubMed
-
- Bianchi DW, Platt LD, Goldberg JD, Abuhamad AZ, Sehnert AJ, Rava RP. Genome-Wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obstet Gynecol. 2012;119(5) - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

