Macrolide therapy in adults and children with non-cystic fibrosis bronchiectasis: a systematic review and meta-analysis
- PMID: 24603554
- PMCID: PMC3946068
- DOI: 10.1371/journal.pone.0090047
Macrolide therapy in adults and children with non-cystic fibrosis bronchiectasis: a systematic review and meta-analysis
Abstract
Background: A systematic review and meta-analysis was conducted to evaluate the efficacy and safety of macrolide therapy in adults and children with bronchiectasis.
Methods: We searched the PUBMED, EMBASE, CENTRAL databases to identify relevant studies. Two reviewers evaluated the studies and extracted data independently. The primary outcome was the number of bronchiectasis exacerbations. Secondary outcomes included exacerbation-related admissions, quality of life (QoL), spirometry, 6-minute walk test (6MWT) and adverse events.
Results: Nine eligible trials with 559 participants were included. Six were conducted on adults, and the remaining on children. Macrolide therapy significantly reduced the number of patients experiencing one or more exacerbation in adults [risk ratio (RR) = 0.59; 95% CI, 0.40 to 0.86; P = 0.006; I2 = 65%] and children [RR = 0.86; 95% CI, 0.75-0.99; P = 0.04; I2 = 0%], but not the number of patients with admissions for exacerbation. Macrolide therapy was also associated with reduced frequency of exacerbations in adults (RR = 0.42; 95% CI, 0.29 to 0.61; P<0.001; I2 = 64%) and children (RR = 0.50; 95% CI, 0.35 to 0.71; P<0.001). Pooled analyses suggested that spirometry, including FEV1 and FVC, were significantly improved in adults but not in children. Macrolide therapy improved the QoL (WMD, -6.56; 95% CI, -11.99 to -1.12; P = 0.02; I2 = 86%) but no significant difference in 6MWT (WMD, 4.15; 95% CI, -11.83 to 20.13; P = 0.61; I2 = 31%) and the overall adverse events (RR, 0.96; 95% CI, 0.82 to 1.13; P = 0.66; I2 = 0%) in adults. However, reports of diarrhea and abdominal discomforts were higher with macrolide therapy.
Conclusions: Macrolide maintenance therapy, both in adults and children, was effective and safe in reducing bronchiectasis exacerbations, but not the admissions for exacerbations. In addition, macrolide administration in adults was associated with improvement in QoL and spirometry, but not 6WMT. Future studies are warranted to verify the optimal populations and clarify its potential effects on antimicrobial resistance.
Conflict of interest statement
Figures



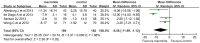

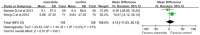
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

