A randomized phase III study evaluating the efficacy and safety of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy
- PMID: 24603643
- PMCID: PMC4071754
- DOI: 10.1093/annonc/mdu101
A randomized phase III study evaluating the efficacy and safety of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy
Abstract
Background: Antiemetic guidelines recommend co-administration of agents that target multiple molecular pathways involved in emesis to maximize prevention and control of chemotherapy-induced nausea and vomiting (CINV). NEPA is a new oral fixed-dose combination of 300 mg netupitant, a highly selective NK1 receptor antagonist (RA) and 0.50 mg palonosetron (PALO), a pharmacologically and clinically distinct 5-HT3 RA, which targets dual antiemetic pathways.
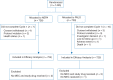
Patients and methods: This multinational, randomized, double-blind, parallel group phase III study (NCT01339260) in 1455 chemotherapy-naïve patients receiving moderately emetogenic (anthracycline-cyclophosphamide) chemotherapy evaluated the efficacy and safety of a single oral dose of NEPA versus a single oral dose (0.50 mg) of PALO. All patients also received oral dexamethasone (DEX) on day 1 only (12 mg in the NEPA arm and 20 mg in the PALO arm). The primary efficacy end point was complete response (CR: no emesis, no rescue medication) during the delayed (25-120 h) phase in cycle 1.
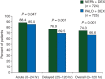
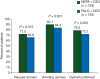
Results: The percentage of patients with CR during the delayed phase was significantly higher in the NEPA group compared with the PALO group (76.9% versus 69.5%; P = 0.001), as were the percentages in the overall (0-120 h) (74.3% versus 66.6%; P = 0.001) and acute (0-24 h) (88.4% versus 85.0%; P = 0.047) phases. NEPA was also superior to PALO during the delayed and overall phases for all secondary efficacy end points of no emesis, no significant nausea and complete protection (CR plus no significant nausea). NEPA was well tolerated with a similar safety profile as PALO.
Conclusions: NEPA plus a single dose of DEX was superior to PALO plus DEX in preventing CINV following moderately emetogenic chemotherapy in acute, delayed and overall phases of observation. As a fixed-dose antiemetic drug combination, NEPA along with a single dose of DEX on day 1 offers guideline-based prophylaxis with a convenient, single-day treatment.
Keywords: CINV; NEPA; moderately emetogenic; netupitant; neurokinin-1 receptor antagonist; palonosetron.
© The Author 2014. Published by Oxford University Press on behalf of the European Society for Medical Oncology.
Figures
Comment in
-
Chemotherapy: NEPA-a single oral dose providing effective prevention of chemotherapy-induced nausea and vomiting.Nat Rev Clin Oncol. 2014 Jul;11(7):377. doi: 10.1038/nrclinonc.2014.92. Epub 2014 Jun 3. Nat Rev Clin Oncol. 2014. PMID: 24889769 No abstract available.
References
-
- Frame DG. Best practice management of CINV in oncology patients: I. physiology and treatment of CINV. Multiple neurotransmitters and receptors and the need for combination therapeutic approaches. J Support Oncol. 2010;8(1):5–9. - PubMed
-
- Feyer P, Jordan K. Update and new trends in antiemetic therapy: the continuing need for novel therapies. Ann Oncol. 2011;22(1):30–38. - PubMed
-
- Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;21(5):232–243. - PubMed
-
- Warr DG, Hesketh PJ, Gralla RJ, et al. Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol. 2005;23(12):2822–2830. - PubMed
-
- Aapro M, Molassiotis A, Dicato M, et al. The effect of guideline-consistent antiemetic therapy on chemotherapy-induced nausea and vomiting (CINV): the Pan European Emesis Registry (PEER) Ann Oncol. 2012;23(8):1986–1992. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials