Hybrid supercapacitor-battery materials for fast electrochemical charge storage
- PMID: 24603843
- PMCID: PMC3945924
- DOI: 10.1038/srep04315
Hybrid supercapacitor-battery materials for fast electrochemical charge storage
Abstract
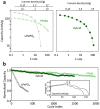
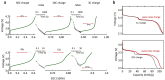
High energy and high power electrochemical energy storage devices rely on different fundamental working principles--bulk vs. surface ion diffusion and electron conduction. Meeting both characteristics within a single or a pair of materials has been under intense investigations yet, severely hindered by intrinsic materials limitations. Here, we provide a solution to this issue and present an approach to design high energy and high power battery electrodes by hybridizing a nitroxide-polymer redox supercapacitor (PTMA) with a Li-ion battery material (LiFePO4). The PTMA constituent dominates the hybrid battery charge process and postpones the LiFePO4 voltage rise by virtue of its ultra-fast electrochemical response and higher working potential. We detail on a unique sequential charging mechanism in the hybrid electrode: PTMA undergoes oxidation to form high-potential redox species, which subsequently relax and charge the LiFePO4 by an internal charge transfer process. A rate capability equivalent to full battery recharge in less than 5 minutes is demonstrated. As a result of hybrid's components synergy, enhanced power and energy density as well as superior cycling stability are obtained, otherwise difficult to achieve from separate constituents.
Conflict of interest statement
UCL has filed a patent on some of the intellectual property disclosed by this paper.
Figures



References
-
- Tarascon J. & Armand M. Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001). - PubMed
-
- Armand M. & Tarascon J. M. Building better batteries. Nature 451, 652–657 (2008). - PubMed
-
- Choi N.-S. et al. Challenges facing lithium batteries and electrical double-layer capacitors. Angew. Chem. Intl. Ed. 51, 9994–10024 (2012). - PubMed
-
- van Schalkwijk W. A. & Scrosati B. Advances in Lithium-Ion Batteries (Kluwer Academic/Plenum 2002).
-
- Zhang H., Yu X. & Braun P. Three-dimensional bicontinuous ultrafast-charge and -discharge bulk battery electrodes. Nat. Nanotech. 6, 277–281 (2011). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

