A polysaccharide virulence factor from Aspergillus fumigatus elicits anti-inflammatory effects through induction of Interleukin-1 receptor antagonist
- PMID: 24603878
- PMCID: PMC3946377
- DOI: 10.1371/journal.ppat.1003936
A polysaccharide virulence factor from Aspergillus fumigatus elicits anti-inflammatory effects through induction of Interleukin-1 receptor antagonist
Abstract
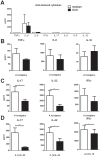
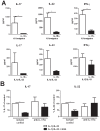
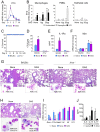
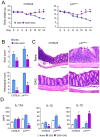
The galactosaminogalactan (GAG) is a cell wall component of Aspergillus fumigatus that has potent anti-inflammatory effects in mice. However, the mechanisms responsible for the anti-inflammatory property of GAG remain to be elucidated. In the present study we used in vitro PBMC stimulation assays to demonstrate, that GAG inhibits proinflammatory T-helper (Th)1 and Th17 cytokine production in human PBMCs by inducing Interleukin-1 receptor antagonist (IL-1Ra), a potent anti-inflammatory cytokine that blocks IL-1 signalling. GAG cannot suppress human T-helper cytokine production in the presence of neutralizing antibodies against IL-1Ra. In a mouse model of invasive aspergillosis, GAG induces IL-1Ra in vivo, and the increased susceptibility to invasive aspergillosis in the presence of GAG in wild type mice is not observed in mice deficient for IL-1Ra. Additionally, we demonstrate that the capacity of GAG to induce IL-1Ra could also be used for treatment of inflammatory diseases, as GAG was able to reduce severity of an experimental model of allergic aspergillosis, and in a murine DSS-induced colitis model. In the setting of invasive aspergillosis, GAG has a significant immunomodulatory function by inducing IL-1Ra and notably IL-1Ra knockout mice are completely protected to invasive pulmonary aspergillosis. This opens new treatment strategies that target IL-1Ra in the setting of acute invasive fungal infection. However, the observation that GAG can also protect mice from allergy and colitis makes GAG or a derivative structure of GAG a potential treatment compound for IL-1 driven inflammatory diseases.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Chai LY, Hsu LY (2011) Recent advances in invasive pulmonary aspergillosis. Curr Opin Pulm Med 17: 160–166. - PubMed
-
- Romani L (2011) Immunity to fungal infections. Nat Rev Immunol 11: 275–288. - PubMed
-
- Chai LY, Netea MG, Vonk AG, Kullberg BJ (2009) Fungal strategies for overcoming host innate immune response. Med Mycol 47: 227–236. - PubMed
-
- Aimanianda V, Bayry J, Bozza S, Kniemeyer O, Perruccio K, et al. (2009) Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 460: 1117–1121. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

