Exogenous hydrogen sulfide (H2S) protects alveolar growth in experimental O2-induced neonatal lung injury
- PMID: 24603989
- PMCID: PMC3946270
- DOI: 10.1371/journal.pone.0090965
Exogenous hydrogen sulfide (H2S) protects alveolar growth in experimental O2-induced neonatal lung injury
Abstract
Background: Bronchopulmonary dysplasia (BPD), the chronic lung disease of prematurity, remains a major health problem. BPD is characterized by impaired alveolar development and complicated by pulmonary hypertension (PHT). Currently there is no specific treatment for BPD. Hydrogen sulfide (H2S), carbon monoxide and nitric oxide (NO), belong to a class of endogenously synthesized gaseous molecules referred to as gasotransmitters. While inhaled NO is already used for the treatment of neonatal PHT and currently tested for the prevention of BPD, H2S has until recently been regarded exclusively as a toxic gas. Recent evidence suggests that endogenous H2S exerts beneficial biological effects, including cytoprotection and vasodilatation. We hypothesized that H2S preserves normal alveolar development and prevents PHT in experimental BPD.
Methods: We took advantage of a recently described slow-releasing H2S donor, GYY4137 (morpholin-4-ium-4-methoxyphenyl(morpholino) phosphinodithioate) to study its lung protective potential in vitro and in vivo.
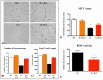
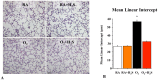
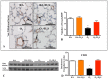
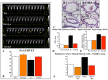
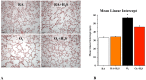
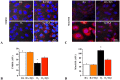
Results: In vitro, GYY4137 promoted capillary-like network formation, viability and reduced reactive oxygen species in hyperoxia-exposed human pulmonary artery endothelial cells. GYY4137 also protected mitochondrial function in alveolar epithelial cells. In vivo, GYY4137 preserved and restored normal alveolar growth in rat pups exposed from birth for 2 weeks to hyperoxia. GYY4137 also attenuated PHT as determined by improved pulmonary arterial acceleration time on echo-Doppler, pulmonary artery remodeling and right ventricular hypertrophy. GYY4137 also prevented pulmonary artery smooth muscle cell proliferation.
Conclusions: H2S protects from impaired alveolar growth and PHT in experimental O2-induced lung injury. H2S warrants further investigation as a new therapeutic target for alveolar damage and PHT.
Conflict of interest statement
Figures

Similar articles
-
Systemic hydrogen sulfide administration partially restores normal alveolarization in an experimental animal model of bronchopulmonary dysplasia.Am J Physiol Lung Cell Mol Physiol. 2014 Apr 1;306(7):L684-97. doi: 10.1152/ajplung.00361.2013. Epub 2014 Feb 7. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 24508731
-
Activation of Akt protects alveoli from neonatal oxygen-induced lung injury.Am J Respir Cell Mol Biol. 2011 Feb;44(2):146-54. doi: 10.1165/rcmb.2009-0182OC. Epub 2010 Mar 26. Am J Respir Cell Mol Biol. 2011. PMID: 20348209
-
Biological Effects of Morpholin-4-Ium 4 Methoxyphenyl (Morpholino) Phosphinodithioate and Other Phosphorothioate-Based Hydrogen Sulfide Donors.Antioxid Redox Signal. 2020 Jan 10;32(2):145-158. doi: 10.1089/ars.2019.7896. Antioxid Redox Signal. 2020. PMID: 31642346 Review.
-
Hydrogen Sulfide Protects Retinal Ganglion Cells Against Glaucomatous Injury In Vitro and In Vivo.Invest Ophthalmol Vis Sci. 2017 Oct 1;58(12):5129-5141. doi: 10.1167/iovs.17-22200. Invest Ophthalmol Vis Sci. 2017. PMID: 28986598
-
GYY4137, a novel water-soluble, H2S-releasing molecule.Methods Enzymol. 2015;554:143-67. doi: 10.1016/bs.mie.2014.11.014. Epub 2015 Jan 10. Methods Enzymol. 2015. PMID: 25725521 Review.
Cited by
-
Endothelial colony-forming cell therapy for heart morphological changes after neonatal high oxygen exposure in rats, a model of complications of prematurity.Physiol Rep. 2018 Nov;6(22):e13922. doi: 10.14814/phy2.13922. Physiol Rep. 2018. PMID: 30485704 Free PMC article.
-
H2S as a potential defense against COVID-19?Am J Physiol Cell Physiol. 2020 Aug 1;319(2):C244-C249. doi: 10.1152/ajpcell.00187.2020. Epub 2020 Jun 9. Am J Physiol Cell Physiol. 2020. PMID: 32515982 Free PMC article. Review.
-
Stem Cells and Their Mediators - Next Generation Therapy for Bronchopulmonary Dysplasia.Front Med (Lausanne). 2015 Jul 30;2:50. doi: 10.3389/fmed.2015.00050. eCollection 2015. Front Med (Lausanne). 2015. PMID: 26284246 Free PMC article. Review.
-
SIRT3 Mediates the Antioxidant Effect of Hydrogen Sulfide in Endothelial Cells.Antioxid Redox Signal. 2016 Feb 20;24(6):329-43. doi: 10.1089/ars.2015.6331. Epub 2015 Nov 10. Antioxid Redox Signal. 2016. PMID: 26422756 Free PMC article.
-
Stem Cell Therapy and Hydrogen Sulfide: Conventional or Nonconventional Mechanisms of Action?Shock. 2020 Jun;53(6):737-743. doi: 10.1097/SHK.0000000000001420. Shock. 2020. PMID: 31348146 Free PMC article.
References
-
- Iams JD, Romero R, Culhane JF, Goldenberg RL (2008) Primary, secondary, and tertiary interventions to reduce the morbidity and mortality of preterm birth. Lancet 371: 164–175. - PubMed
-
- Shah PS, Sankaran K, Aziz K, Allen AC, Seshia M, et al. (2012) Outcomes of preterm infants <29 weeks gestation over 10-year period in Canada: a cause for concern? J Perinatol 32: 132–138. - PubMed
-
- Kinsella JP, Greenough A, Abman SH (2006) Bronchopulmonary dysplasia. Lancet 367: 1421–1431. - PubMed
-
- Kotecha SJ, Edwards MO, Watkins WJ, Henderson AJ, Paranjothy S, et al. (2013) Effect of preterm birth on later FEV1: a systematic review and meta-analysis. Thorax 68: 760–766. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

