Protocol for the ProCare Trial: a phase II randomised controlled trial of shared care for follow-up of men with prostate cancer
- PMID: 24604487
- PMCID: PMC3948582
- DOI: 10.1136/bmjopen-2014-004972
Protocol for the ProCare Trial: a phase II randomised controlled trial of shared care for follow-up of men with prostate cancer
Abstract
Introduction: Men with prostate cancer require long-term follow-up to monitor disease progression and manage common adverse physical and psychosocial consequences of treatment. There is growing recognition of the potential role of primary care in cancer follow-up. This paper describes the protocol for a phase II multisite randomised controlled trial of a novel model of shared care for the follow-up of men after completing treatment for low-moderate risk prostate cancer.
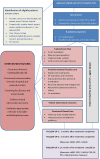
Methods and analysis: The intervention is a shared care model of follow-up visits in the first 12 months after completing treatment for prostate cancer with the following specific components: a survivorship care plan, general practitioner (GP) management guidelines, register and recall systems, screening for distress and unmet needs and patient information resources. Eligible men will have completed surgery and/or radiotherapy for low-moderate risk prostate cancer within the previous 8 weeks and have a GP who consents to participate. Ninety men will be randomised to the intervention or current hospital follow-up care. Study outcome measures will be collected at baseline, 3, 6 and 12 months and include anxiety, depression, unmet needs, prostate cancer-specific quality of life and satisfaction with care. Clinical processes and healthcare resource usage will also be measured. The principal emphasis of the analysis will be on obtaining estimates of the treatment effect size and assessing feasibility in order to inform the design of a subsequent phase III trial.
Ethics and dissemination: Ethics approval has been granted by the University of Western Australia and from all hospital recruitment sites in Western Australia and Victoria.
Results: of this phase II trial will be reported in peer-reviewed publications and in conference presentations.
Trial registration: Australian New Zealand Clinical Trial Registry ACTRN12610000938000.
Keywords: Primary Care.
References
-
- Center MM, Jemal A, Lortet-Tieulent J, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol 2012;61:1079–92 - PubMed
-
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893–917 - PubMed
-
- Cancer in Australia: an overview. Canberra: Australian Institute of Health and Welfare, 2012
-
- Cancer incidence projections: Australia, 2011–2020. Canberra: Australian Institute of Health and Welfare, 2012
-
- Smith BD, Smith GL, Hurria A, et al. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol 2009;27:2758–65 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical