Targeted next-generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas
- PMID: 24604757
- PMCID: PMC4057302
- DOI: 10.1002/path.4344
Targeted next-generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas
Abstract
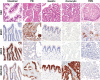
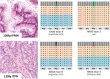
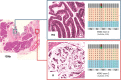
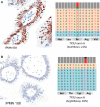
Intraductal neoplasms are important precursors to invasive pancreatic cancer and provide an opportunity to detect and treat pancreatic neoplasia before an invasive carcinoma develops. The diagnostic evaluation of these lesions is challenging, as diagnostic imaging and cytological sampling do not provide accurate information on lesion classification, the grade of dysplasia or the presence of invasion. Moreover, the molecular driver gene mutations of these precursor lesions have yet to be fully characterized. Fifty-two intraductal papillary neoplasms, including 48 intraductal papillary mucinous neoplasms (IPMNs) and four intraductal tubulopapillary neoplasms (ITPNs), were subjected to the mutation assessment in 51 cancer-associated genes, using ion torrent semiconductor-based next-generation sequencing. P16 and Smad4 immunohistochemistry was performed on 34 IPMNs and 17 IPMN-associated carcinomas. At least one somatic mutation was observed in 46/48 (96%) IPMNs; 29 (60%) had multiple gene alterations. GNAS and/or KRAS mutations were found in 44/48 (92%) of IPMNs. GNAS was mutated in 38/48 (79%) IPMNs, KRAS in 24/48 (50%) and these mutations coexisted in 18/48 (37.5%) of IPMNs. RNF43 was the third most commonly mutated gene and was always associated with GNAS and/or KRAS mutations, as were virtually all the low-frequency mutations found in other genes. Mutations in TP53 and BRAF genes (10% and 6%) were only observed in high-grade IPMNs. P16 was lost in 7/34 IPMNs and 9/17 IPMN-associated carcinomas; Smad4 was lost in 1/34 IPMNs and 5/17 IPMN-associated carcinomas. In contrast to IPMNs, only one of four ITPNs had detectable driver gene (GNAS and NRAS) mutations. Deep sequencing DNA from seven cyst fluid aspirates identified 10 of the 13 mutations detected in their associated IPMN. Using next-generation sequencing to detect cyst fluid mutations has the potential to improve the diagnostic and prognostic stratification of pancreatic cystic neoplasms.
Keywords: IPMN; biomarkers; next-generation sequencing (NGS); pancreatic tumours.
© 2014 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Figures


References
-
- Adsay NV, Fukushima N, Furukawa T. Intraductal neoplasms of the pancreas. In: Bosman FT, Carneiro F, Hruban RH, et al., editors. WHO Classification of Tumours of the Digestive System. 4th edn. Lyon: IARC; 2010.
-
- Luttges J, Zamboni G, Longnecker D, et al. The immunohistochemical mucin expression pattern distinguishes different types of intraductal papillary mucinous neoplasms of the pancreas and determines their relationship to mucinous noncystic carcinoma and ductal adenocarcinoma. Am J Surg Pathol. 2001;25::942–948. - PubMed
-
- Chadwick B, Willmore-Payne C, Tripp S, et al. Histologic, immunohistochemical, and molecular classification of 52 IPMNs of the pancreas. Appl Immunohistochem Mol Morphol. 2009;17::31–39. - PubMed
-
- Mohri D, Asaoka Y, Ijichi H, et al. Different subtypes of intraductal papillary mucinous neoplasm in the pancreas have distinct pathways to pancreatic cancer progression. J Gastroenterol. 2012;47::203–213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

