Assessment of inhibited alveolar-capillary membrane structural development and function in bronchopulmonary dysplasia
- PMID: 24604816
- PMCID: PMC3999962
- DOI: 10.1002/bdra.23226
Assessment of inhibited alveolar-capillary membrane structural development and function in bronchopulmonary dysplasia
Abstract
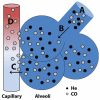
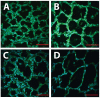
Bronchopulmonary dysplasia (BPD) is a chronic lung disease of extreme prematurity and is defined clinically by dependence on supplemental oxygen due to impaired gas exchange. Optimal gas exchange is dependent on the development of a sufficient surface area for diffusion. In the mammalian lung, rapid acquisition of distal lung surface area is accomplished in neonatal and early adult life by means of vascularization and secondary septation of distal lung airspaces. Extreme preterm birth interrupts secondary septation and pulmonary capillary development and ultimately reduces the efficiency of the alveolar-capillary membrane. Although pulmonary health in BPD infants rapidly improves over the first few years, persistent alveolar-capillary membrane dysfunction continues into adolescence and adulthood. Preventative therapies have been largely ineffective, and therapies aimed at promoting normal development of the air-blood barrier in infants with established BPD remain largely unexplored. The purpose of this review will be: (1) to summarize the histological evidence of aberrant alveolar-capillary membrane development associated with extreme preterm birth and BPD, (2) to review the clinical evidence assessing the long-term impact of BPD on alveolar-capillary membrane function, and (3) to discuss the need to develop and incorporate direct measurements of functional gas exchange into clinically relevant animal models of inhibited alveolar development.
Keywords: bronchopulmonary dysplasia; diffusing capacity; hyperoxia; lung function; mouse; neonatal lung morphogenesis.
Copyright © 2014 Wiley Periodicals, Inc.
Figures
References
-
- Angus GE, Thurlbeck WM. Number of alveoli in the human lung. J Appl Physiol. 1972;32:483–485. - PubMed
-
- Aukland SM, Rosendahl K, Owens CM, et al. Neonatal bronchopulmonary dysplasia predicts abnormal pulmonary HRCT scans in long-term survivors of extreme preterm birth. Thorax. 2009;64:405–410. - PubMed
-
- Balasubramaniam V, Mervis CF, Maxey AM, et al. Hyperoxia reduces bone marrow, circulating, and lung endothelial progenitor cells in the developing lung: implications for the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1073–L1084. - PubMed
-
- Ballard PL. Hormonal regulation of pulmonary surfactant. Endocr Rev. 1989;10:165–181. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

