Improving engraftment and immune reconstitution in umbilical cord blood transplantation
- PMID: 24605111
- PMCID: PMC3932655
- DOI: 10.3389/fimmu.2014.00068
Improving engraftment and immune reconstitution in umbilical cord blood transplantation
Abstract
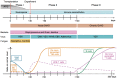
Umbilical cord blood (UCB) is an important source of hematopoietic stem cells (HSC) for allogeneic transplantation when HLA-matched sibling and unrelated donors (MUD) are unavailable. Although the overall survival results for UCB transplantation are comparable to the results with MUD, UCB transplants are associated with slow engraftment, delayed immune reconstitution, and increased opportunistic infections. While this may be a consequence of the lower cell dose in UCB grafts, it also reflects the relative immaturity of cord blood. Furthermore, limited cell numbers and the non-availability of donor lymphocyte infusions currently prevent the use of post-transplant cellular immunotherapy to boost donor-derived immunity to treat infections, mixed chimerism, and disease relapse. To further develop UCB transplantation, many strategies to enhance engraftment and immune reconstitution are currently under investigation. This review summarizes our current understanding of engraftment and immune recovery following UCB transplantation and why this differs from allogeneic transplants using other sources of HSC. It also provides a comprehensive overview of promising techniques being used to improve myeloid and lymphoid recovery, including expansion, homing, and delivery of UCB HSC; combined use of UCB with third-party donors; isolation and expansion of natural killer cells, pathogen-specific T cells, and regulatory T cells; methods to protect and/or improve thymopoiesis. As many of these strategies are now in clinical trials, it is anticipated that UCB transplantation will continue to advance, further expanding our understanding of UCB biology and HSC transplantation.
Keywords: engraftment; hematopoietic stem cells; immune reconstitution; transplantation; umbilical cord blood.
Figures
References
-
- Gluckman E, Rocha V, Boyer-Chammard A, Locatelli F, Arcese W, Pasquini R, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med (1997) 337(6):373–8110.1056/NEJM199708073370602 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

